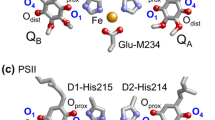
Abstract
Quinones such as ubiquinone are the lipid soluble electron and proton carriers in the membranes of mitochondria, chloroplasts and oxygenic bacteria. Quinones undergo controlled redox reactions bound to specific sites in integral membrane proteins such as the cytochrome bc1 oxidoreductase. The quinone reactions in bacterial photosynthesis are amongst the best characterized, presenting a model to understand how proteins modulate cofactor chemistry. The free energy of ubiquinone redox reactions in aqueous solution and in the QA and QB sites of the bacterial photosynthetic reaction centers (RCs) are compared. In the primary QA site ubiquinone is reduced only to the anionic semiquinone (Q•−) while in the secondary QB site the product is the doubly reduced, doubly protonated quinol (QH2). The ways in which the protein modifies the relative energy of each reduced and protonated intermediate are described. For example, the protein stabilizes Q•− while destabilizing Q= relative to aqueous solution through electrostatic interactions. In addition, kinetic and thermodynamic mechanisms for stabilizing the intermediate semiquinones are compared. Evidence for the protein sequestering anionic compounds by slowing both on and off rates as well as by binding the anion more tightly is reviewed.
Similar content being viewed by others
References
Alexov EG, Gunner MR (1997) Incorporating protein conformational flexibility into the calculation of pH-dependent protein properties. Biophys J 72:2075–2093
Alexov EG, Gunner MR (1999) Calculated protein and proton motions coupled to electron transfer: electron transfer from QA − to QB in bacterial photosynthetic reaction centers. Biochemistry 38:8253–8270
Alexov E, Miksovska J, Baciou L, Schiffer M, Hanson DK, Sebban P, Gunner MR (2000) Modeling the effects of mutations on the free energy of the first electron transfer from QA − to QB in photosynthetic reaction centers. Biochemistry 39:5940–5952
Baker NA (2005) Improving implicit solvent simulations: a Poisson-centric view. Cur Opin Struct Biol 15:137–143
Bashford D, Karplus M (1990) The pKas of ionizable groups in proteins: atomic detail from a continuum electrostatic model. Biochemistry 29:10219–10225
Beroza P, Case D (1996) Including side chain flexibility in continuum electrostatic calculations of protein titration. J Phys Chem 100:20156–20163
Beroza P, Fredkin DR, Okamura MY, Feher G (1991) Protonation of interacting residues in a protein by a Monte Carlo method: application to Lysozyme and the photosynthetic reaction center of Rhodobacter sphaeroides. Proc Natl Acad Sci USA 88:5804–5808
Beroza P, Fredkin DR, Okamura MY, Feher R (1995) Electrostatic calculations of amino acid titration electron transfer, Q− AQB→QAQB −, in the reaction center. Biophys J 68:2233–2250
Bockris JOM, Reddy AKN (1973) Modern electrochemistry, vol. 1. Plenum, New York
Breton J (2004) Absence of large-scale displacement of quinone QB in bacterial photosynthetic reaction centers. Biochemistry 43:3318–3326
Breton J, Boullais C, Mioskowski C, Sebban P, Bacious L, Nabedryk E (2002) Vibrational spectroscopy favors a unique QB binding site at the proximal position in wild-type reaction centers and in the Pro-L209 → Tyr mutant from Rhodobacter sphaeroides. Biochemistry 41:12921–12927
Cape JL, Strahan JR, Lenaeus MJ, Yuknis BA, Le TT, Shepherd JN, Bowman MK, Kramer DM (2005) The respiratory substrate rhodoquinol induces Q-cycle bypass reactions in the yeast cytochrome bc1 complex: mechanistic and physiological implications. J Biol Chem 280:34654–34660
Cape JL, Bowman MK, Kramer DM (2006) Computation of the redox and protonation properties of quinones: towards the prediction of redox cycling natural products. Phytochemistry 67:1781–1788
Cape JL, Bowman MK, Kramer DM (2006) Understanding the cytochrome bc complexes by what they don’t do. The Q-cycle at 30. Trends Plant Sci 11:46–55
Cape JL, Bowman MK, Kramer DM (2007) A semiquinone intermediate generated at the Qo site of the cytochrome bc1 complex: importance for the Q-cycle and superoxide production. Proc Natl Acad Sci USA 104:7887–7892
Churg AK, Warshel A (1986) Control of the redox potential of cytochrome c and microscopic dielectric effects in proteins. Biochemistry 25:1675–1681
Churg AK, Weiss RM, Warshel A, Takano T (1983) On the action of cytochrome c: correlating geometry changes upon oxidation with activation energies of electron transfer. J Phys Chem 87:1683–1694
Diner BA, Schenck CC, DeVitry C (1984) Effect of inhibitors, redox state and isoprenoid chain length on the affinity of ubiquinone for the secondary acceptor binding site in the reaction centers of photosynthetic bacteria. Biochim Biophys Acta 766:9–20
Dutton PL, Leigh JS, Wraight CA (1973) Direct measurement of the midpoint potential of the primary electron acceptor in Rhodopseudomonas spheroides in situ and in the isolated state: some relationships with pH and o-phenanthroline. FEBS Lett 36:169–173
Feher G, Allen JP, Okamura MY, Rees DC (1989) Primary processes in bacterial photosynthesis: structure and function of bacterial photosynthetic reaction centres. Nature 339:111–116
Forquer I, Covian R, Bowman MK, Trumpower B, Kramer DM (2006) Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J Biol Chem 281:38459–38465
Georgescu RE, Alexov EG, Gunner MR (2002) Combining conformational flexibility and continuum electrostatics for calculating pKas in proteins. Biophys J 83:1731–1748
Graige MS, Paddock ML, Bruce JM, Feher G, Okamura MY (1996) Mechanism of proton-coupled electron transfer for quinone (QB) reduction in reaction centers of R. sphaeroides. J Am Chem Soc 118:9005–9016
Graige MS, Feher G, Okamura MY (1998) Conformational gating of the electron transfer reaction QA −QB →QAQB − in bacterial reaction centers of Rhodobacter sphaeroides determined by a driving force assay. Proc Natl Acad Sci USA 95:11679–11684
Graige MS, Paddock ML, Feher G, Okamura MY (1999) Observation of the protonated semiquinone intermediate in isolated reaction centers from Rhodobacter sphaeroides: implications for the mechanism of electron and proton transfer in proteins. Biochemistry 38:11465–11473
Gunner MR (1991) The reaction center protein from purple bacteria: structure and function. Curr Top Bioenerg 16:319–367
Gunner MR, Alexov E (2000) A pragmatic approach to structure based calculation of coupled proton and electron transfer in proteins. Biochim Biophys Acta 1458:63–87
Gunner MR, Dutton PL (1989) Temperature and −ΔG° dependence of the electron transfer from BPh− to QA in reaction center protein from Rhodobacter sphaeroides with different quinones as QA. J Am Chem Soc 111:3400–3412
Gunner MR, Honig B (1991) Electrostatic control of midpoint potentials in the cytochrome subunit of the Rhodopseudomonas viridis reaction center. Proc Natl Acad Sci USA 88:9151–9155
Gunner MR, Nicholls A, Honig B (1996) Electrostatic potentials in Rhodopseudomonas viridis reaction center: implications for the driving force and directionality of electron transfer. J Phys Chem 100:4277–4291
Gunner MR, Alexov E, Torres E, Lipovaca S (1997) The importance of the protein in controlling the electrochemistry of heme metalloproteins: methods of calculation and analysis. J Biol Inorg Chem 2:126–134
Gunner MR, Mao J, Song Y, Kim J (2006) Factors influencing energetics of electron and proton transfers in proteins. What can be learned from calculations. Biochim Biophys Acta 1757:942–968
Haas AH, Lancaster CR (2004) Calculated coupling of transmembrane electron and proton transfer in dihemic quinol:fumarate reductase. Biophys J 87:4298–4315
Hasegawa J-y, Ishida M, Nakatsuji H, Lu Z, Liu H, Yang W (2003) Energetics of the electron transfer from bacteriopheophytin to ubiquinone in the photosynthetic reaction center of Rhodopseudomonas viridis: theoretical study. J Phys Chem B 107:838–847
Heathcote P (2002) Reaction centers: the structure and evolution of biological solar power. Trends Biochem Sci 27:79–86
Honig B, Nicholls A (1995) Classical electrostatics in biology and chemistry. Science 268:1144–1149
Ishikita H, Knapp EW (2004) Variation of Ser-L223 hydrogen bonding with the QB redox state in reaction centers from Rhodobacter sphaeroides. J Am Chem Soc 126:8059–8064
Ishikita H, Morra G, Knapp EW (2003) Redox potential of quinones in photosynthetic reaction centers from Rhodobacter sphaeroides: dependence on protonation of Glu-L212 and Asp-L213. Biochemistry 42:3882–3892
Kassner RJ (1972) Effects of nonpolar environments on the redox potentials of heme complexes. Proc Natl Acad Sci USA 69:2263–2267
Kim J, Mao J, Gunner MR (2005) Are acidic and basic groups in buried proteins predicted to be ionized? J Mol Biol 348:1283–1298
Kleinfeld D, Okamura MY, Feher G (1984a) Electron transfer in reaction centers of Rhodopseudomonas sphaeroides: I. Determination of the charge recombination pathway of D+ QAQB − and free energy and kinetic relations between QA −QB and QAQB −. Biochim Biophys Acta 766:126–140
Kleinfeld D, Okamura MY, Feher G (1984b) Electron-transfer kinetics in photosynthetic reaction centers cooled to cryogenic temperatures in the charge separated state: evidence for light-induced structural changes. Biochemistry 23:5780–5786
Klingen AR, Palsdottir H, Hunte C, Ullmann GM (2007) Redox-linked protonation state changes in cytochrome bc1 identified by Poisson–Boltzmann electrostatics calculations. Biochim Biophys Acta 1767:204–221
Kramer DM, Roberts AG, Muller F, Cape J, Bowman MK (2004) Q-cycle bypass reactions at the Qo site of the cytochrome bc1 (and related) complexes. Methods Enzymol 382:21–45
Lancaster CRD (1998) Ubiquinone reduction and protonation in photosynthetic reaction centres from Rhodopseudomonas viridis: X-ray structures and their functional implications. Biochim Biophys Acta 1365:143–150
Lancaster CRD, Michel H, Honig B, Gunner MR (1996) Calculated coupling of electron and proton transfer in the photosynthetic reaction center of Rhodopseudomonas viridis. Biophys J 70:2469–2492
Li J, Takahashi E, Gunner MR (2000) −ΔG°AB and pH dependence on the electron transfer from P+ QA −QB to P+ QAQB − in Rhodobacter sphaeroides reaction centers. Biochemistry 39:7445–7454
Madeo J, Gunner MR (2005) Modeling binding kinetics at the QA site in bacterial reaction centers. Biochemistry 44:10994–11004
Mallik B, Datta SN (2004) Semiemprical quantum chemical treatment of the standard reduction potentials of quinone and plastoquinone in water. Int J Quant Chem 52:629–649
Mancino LJ, Dean DP, Blankenship RE (1984) Kinetics and thermodynamics of the P870+ QA −>P870+ QB − reaction in isolated reaction centers from the photosynthetic bacterium Rhodopseudomonas sphaeroides. Biochim Biophys Acta 764:46–54
Mao J, Hauser K, Gunner MR (2003) How cytochromes with different folds control heme redox potentials. Biochemistry 42:9829–9840
Marchi M, Gehlen JN, Chandler D, Newton M (1993) Diabatic surfaces and the pathway for primary electron transfer in a photosynthetic reaction center. J Am Chem Soc 115:4178–4190
Mitchell P (1975a) Proton motive redox mechanism of the cytochrome bc1 complex in the respiratory chain: proton motive ubiquinone cycle. FEBS Lett 56:1–6
Mitchell P (1975b) The proton motive Q cycle: a general formulation. FEBS Lett 59:137–139
Moser CC, Page CC, Cogdell RJ, Barber J, Wraight CA, Dutton PL (2003) Length, time, and energy scales of photosystems. Adv Protein Chem 63:71–109
Moser CC, Farid TA, Chobot SE, Dutton PL (2006) Electron tunneling chains of mitochondria. Biochim Biophys Acta 1757:1096–1109
Okamura MY, Paddock ML, Graige MS, Feher G (2000) Proton and electron transfer in bacterial reaction centers. Biochim Biophys Acta 1458:148–163
Osyczka A, Moser CC, Dutton PL (2005) Fixing the Q cycle. Trends Biochem Sci 30:176–182
Parson WW, Chu Z-T, Warshel A (1990) Electrostatic control of charge separation in bacterial photosynthesis. Biochim Biophys Acta 1017:251–272
Pokkuluri PR, Laible PD, Crawford AE, Mayfield JF, Yousef MA, Ginell SL, Hanson DK, Schiffer M (2004) Temperature and cryoprotectant influence secondary quinone binding position in bacterial reaction centers. FEBS Let 43:9909–9917
Prince RC, Dutton PL, Bruce JM (1983) Electrochemistry of ubiquinones. FEBS Lett 160:273–276
Prince RC, Lloyd-Williams P, Bruce JM, Dutton PL (1986) Voltammetric measurements of quinines. Methods Enzymol 125:109–119
Rabenstein B, Ullmann GM, Knapp E-W (1998) Energetics of electron-transfer and protonation reactions of the quinones in the photosynthetic reaction center of Rhodopseudomonas viridis. Biochemistry 37:2488–2495
Rabenstein B, Ullmann GM, Knapp EW (2000) Electron transfer between the quinones in the photosynthetic reaction center and its coupling to conformational changes. Biochemistry 39:10487–10496
Rashin AA, Honig B (1985) Reevaluation of the born model of ion hydration. J Phys Chem 89:5588–5593
Reedy CJ, Gibney BR (2004) Heme protein assemblies. Chem Rev 104:617–649
Remy A, Gerwert K (2003) Coupling of light-induced electron transfer to proton uptake in photosynthesis. Nat Struct Biol 10:637–644
Rich PR (2004) The quinone chemistry of bc complexes. Biochem Biophys Acta 1658:165–171
Rich PR, Bendall DS (1979) A mechanism for the reduction of cytochromes by quinols in solution and its relevance to biological electron transfer reactions. FEBS Lett 105:189–194
Rutherford AW, Evans MCW (1980) Direct measurement of the redox potential of the primary and secondary quinone electron acceptors in Rhodopseudomonas sphaeroides (wild-type) by EPR spectrometry. FEBS Lett 110:257–261
Saraste M (1999) Oxidative phosphorylation at the fin de siecle. Science 283:1488–1493
Schutz CN, Warshel A (2001) What are the “dielectric constants” of proteins and how to validate electrostatic models? Proteins Struct Funct Genet 44:400–417
Sham YY, Chu ZT, Warshel A (1997) Consistent calculations of pKa’s of ionizable residues in proteins: semi-microscopic and microscopic approaches. J Phys Chem 101:4458–4472
Sham Y, Muegge I, Warshel A (1999) Simulating proton translocations in proteins: probing proton transfer pathways in the Rhodobacter sphaeroides reaction center. Proteins Struct Funct Genet 36:484–500
Shurki A, Strajbl M, Schutz CN, Warshel A (2004) Electrostatic basis for bioenergetics. Methods Enzymol 380:52–84
Simonson T (2001) Macromolecular electrostatics: continuum models and their growing pains. Curr Opin Struct Biol 11:243–252
Stowell MHB, McPhillips TM, Rees DC, Soltis SM, Abresch E, Feher G (1997) Light-induced structural changes in photosynthetic reaction center: implications for mechanism of electron–proton transfer. Science 276:812–816
Swallow AJ (1982) Physical chemistry of semiquinones. In: Trumpower BL (ed) Function of quinones in energy conserving systems. Academic, New York, pp 59–72
Ullmann GM, Knapp EW (1999) Electrostatic models for computing protonation and redox equilibria in proteins. Eur Biophys J 28:533–551
Voigt P, Knapp EW (2003) Tuning heme redox potentials in the cytochrome c subunit of photosynthetic reaction centers. J Biol Chem 278:51993–52001
Warshel A, Russell ST (1984) Calculations of electrostatic interactions in biological systems and in solutions. Q Rev Biophys 17:283–422
Woodbury NW, Allen JP (1995) The pathway, kinetics and thermodynamics of electron transfer in wild type and mutant reaction centers of purple nonsulfur bacteria. In: Blankenship RE, Madigan MT, Bauer CE (eds) Anoxygenic photosynthetic bacteria. Kluwer, Dordrecht
Woodbury NW, Parson WW, Gunner MR, Prince RC, Dutton PL (1986) Radical-pair energetics and decay mechanisms in reaction center containing anthraquinones or benzoquinones in place of ubiquinone. Biochim Biophys Acta 851:6–22
Wraight CA (1979) Electron acceptors of bacterial photosynthetic reaction centers II. H+ binding coupled to secondary electron transfer in the quinone acceptor complex. Biochim Biophys Acta 548:309–327
Wraight CA (2004) Proton and electron transfer in the acceptor quinone complex of photosynthetic reaction centers from Rhodobacter sphaeroides. Front Biosci 9:309–337
Xu Q, Gunner MR (2000) Temperature dependence of the free energy, enthalpy and entropy of P+ QA − charge recombination in photosynthetic reaction centers. J Phys Chem B 104:8035–8043
Xu Q, Gunner MR (2001) Trapping conformational intermediate states in the reaction center protein from photosynthetic bacteria. Biochemistry 40:3232–3241
Xu Q, Baciou L, Sebban P, Gunner MR (2002) Exploring the energy landscape for QA − to QB electron transfer in bacterial photosynthetic reaction centers: effect of substrate position and tail length on the conformational gating step. Biochemistry 41:10021–10025
Yang A-S, Gunner MR, Sampogna R, Sharp K, Honig B (1993) On the calculation of pKa’s in proteins. Proteins Struct Funct Genet 15:252–265
You TJ, Bashford D (1995) Conformation and hydrogen ion titration of proteins: a continuum electrostatic model with conformational flexibility. Biophys J 69:1721–1733
Zhang H, Osyczka A, Dutton PL, Moser CC (2007) Exposing the complex III Qo semiquinone radical. Biochim Biophys Acta 1767:883–887
Zhu Z, Gunner MR (2005) Energetics of quinone-dependent electron and proton transfers in Rhodobacter sphaeroides photosynthetic reaction centers. Biochemistry 44:82–96
Zheng Z, Gunner MR (2008) Analysis of the electrochemistry of hemes with Ems spanning 800 mV. Proteins (in press)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunner, M.R., Madeo, J. & Zhu, Z. Modification of quinone electrochemistry by the proteins in the biological electron transfer chains: examples from photosynthetic reaction centers. J Bioenerg Biomembr 40, 509–519 (2008). https://doi.org/10.1007/s10863-008-9179-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-008-9179-1