Abstract
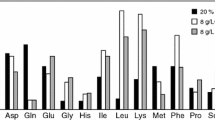
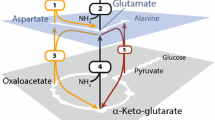
An improved expression protocol is proposed for amino acid type-specific [13C], [15N]-isotope labeling of proteins in baculovirus-infected (BV) insect cell cultures. This new protocol modifies the methods published by Gossert et al. (J Biomol NMR 51(4):449–456, 2011) and provides efficient incorporation of isotopically labeled amino acids, with similar yields per L versus unlabeled expression in rich media. Gossert et al. identified the presence of unlabeled amino acids in the yeastolate of the growth medium as a major limitation in isotope labeling using BV-infected insect cells. By reducing the amount of yeastolate in the growth medium ten-fold, a significant improvement in labeling efficiency was demonstrated, while maintaining good protein expression yield. We report an alternate approach to improve isotope labeling efficiency using BV-infected insect cells namely by replacing the yeast extracts in the medium with dialyzed yeast extracts to reduce the amount of low molecular weight peptides and amino acids. We report the residual levels of amino acids in various media formulations and the amino acid consumption during fermentation, as determined by NMR. While direct replacement of yeastolate with dialyzed yeastolate delivered moderately lower isotope labeling efficiencies compared to the use of ten-fold diluted undialized yeastolate, we show that the use of dialyzed yeastolate combined with a ten-fold dilution delivered enhanced isotope labeling efficiency and at least a comparable level of protein expression yield, all at a scale which economizes use of these costly reagents.


Similar content being viewed by others
References
Assenberg R, Wan PT, Geisse S, Mayr LM (2013) Advances in recombinant protein expression for use in pharmaceutical research. Curr Opin Struct Biol 23(3):393–402
Bellizzi, J. J., III, J. Widom, C. W. Kemp, J. Clardy (1999). Producing selenomethionine-labeled proteins with a baculovirus expression vector system. Structure 7(11): R263–R267
Bruggert M, Rehm T, Shanker S, Georgescu J, Holak TA (2003) A novel medium for expression of proteins selectively labeled with 15 N-amino acids in Spodoptera frugiperda (Sf9) insect cells. J Biomol NMR 25(4):335–348
Creemers AF, Klaassen CH, Bovee-Geurts PH, Kelle R, Kragl U, Raap J, de Grip WJ, Lugtenburg J, de Groot HJ (1999) Solid state 15 N NMR evidence for a complex Schiff base counterion in the visual G-protein-coupled receptor rhodopsin. BioChemistry 38(22):7195–7199
Cristiani, C (2003) Tailoring protein kinases for structural studies. Poster, 6th Annual Meeting Baculovirus and Insect Cell Culture.
DeLange F, Klaassen CH, Wallace-Williams SE, Bovee-Geurts PH, Liu XM, DeGrip WJ, Rothschild KJ (1998) Tyrosine structural changes detected during the photoactivation of rhodopsin. J Biol Chem 273(37):23735–23739
Fremont DH, Crawford F, Marrack P, Hendrickson WA, Kappler J (1998) Crystal structure of mouse H2-M. Immunity 9(3):385–393
Gossert, A. D. and W. Jahnke (2012) Isotope labeling in insect cells. Adv Exp Med Biol 992:179–196
Gossert AD, Hinniger A, Gutmann S, Jahnke W, Strauss A, Fernandez C (2011) A simple protocol for amino acid type selective isotope labeling in insect cells with improved yields and high reproducibility. J Biomol NMR 51(4):449–456
Jarvis, D. L. (2009). Baculovirus-insect cell expression systems. Methods Enzymol 463:191–222
Kelly, B. J., L. A. King, R. D. Possee (2007). Introduction to baculovirus molecular biology.” Methods Mol Biol 388:25–53
Lipovsek, D. (2010). Adnectins: engineered target-binding protein therapeutics. Protein Eng Des Sel 24(1–2):3–9
Maiorella B, Inlow D, Shauger A, Harano D (1988) Large-scale insect cell-culture for recombinant protein production. Bio/Technology 6(12):1406–1410
Meola A, Deville C, Jeffers SA, Guardado-Calvo P, Vasiliauskaite I, Sizun C, Girard-Blanc C, Malosse C, van Heijenoort C, Chamot-Rooke J, Krey T, Guittet E, Petres S, Rey FA, Bontems F (2014) Robust and low cost uniform 15 N-labeling of proteins expressed in Drosophila S2 cells and Spodoptera frugiperda Sf9 cells for NMR applications. J Struct Biol 188(1):71–78
Passarelli, A. L. and L. A. Guarino (2007) Baculovirus late and very late gene regulation. Curr. Drug Targets 8(10):1103–1115
Schlaeger EJ (1996) Medium design for insect cell culture. CytoTechnology 20(1–3):57–70
Sitarska A, Skora L, Klopp J, Roest S, Fernandez C, Shrestha B, Gossert AD (2015) Affordable uniform isotope labeling with 2 H, 13 C and 15 N in insect cells. J Biomol NMR 62(2):191–197
Strauss A, Bitsch F, Cutting B, Fendrich G, Graff P, Liebetanz J, Zurini M, Jahnke W (2003) Amino-acid-type selective isotope labeling of proteins expressed in baculovirus-infected insect cells useful for NMR studies. J Biomol NMR 26(4):367–372
Strauss A, Bitsch F, Fendrich G, Graff P, Knecht R, Meyhack B, Jahnke W (2005) Efficient uniform isotope labeling of Abl kinase expressed in baculovirus-infected insect cells. J Biomol NMR 31(4):343–349
Walton WJ, Kasprzak AJ, Hare JT, Logan TM (2006) An economic approach to isotopic enrichment of glycoproteins expressed from Sf9 insect cells. J Biomol NMR 36(4):225–233
Weiss, S. A., G. C. Smith, S. S. Kalter, J. L. Vaughn (1981). Improved method for the production of insect cell cultures in large volume. In Vitro 17(6):495–502
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wei, H., Xie, D. et al. An improved protocol for amino acid type-selective isotope labeling in insect cells. J Biomol NMR 68, 237–247 (2017). https://doi.org/10.1007/s10858-017-0117-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-017-0117-6