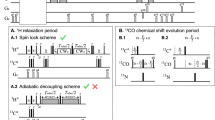
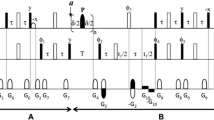
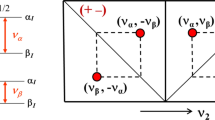
Abstract
The presence of dipole-dipole cross-correlated relaxation as well as unresolved E.COSY effects adversely impacts the accuracy of 1 J NH splittings measured from gradient-enhanced IPAP-HSQC spectra. For isotropic samples, the size of the systematic errors caused by these effects depends on the values of 2 J NHα , 3 J NHβ and 3 J HNHα . Insertion of band-selective 1H decoupling pulses in the IPAP-HSQC experiment eliminates these systematic errors and for the protein GB3 yields 1 J NH splittings that agree to within a root-mean-square difference of 0.04 Hz with values measured for perdeuterated GB3. Accuracy of the method is also highlighted by a good fit to the GB3 structure of the 1H-15N RDCs extracted from the minute differences in 1JNH splitting measured at 500 and 750 MHz 1H frequencies, resulting from magnetic susceptibility anisotropy. A nearly complete set of 2 J NHα couplings was measured in GB3 in order to evaluate whether the impact of cross-correlated relaxation is dominated by the 15N–1Hα or 15N–1Hβ dipolar interaction. As expected, we find that 2 J NHα ≤ 2 Hz, with values in the α-helix (0.86 ± 0.52 Hz) slightly larger than in β-sheet (0.66 ± 0.26 Hz). Results indicate that under isotropic conditions, N–HN/N–Hβ cross-correlated relaxation often dominates. Unresolved E.COSY effects under isotropic conditions involve 3 J HNHα and J NHα , but when weakly aligned any aliphatic proton proximate to both N and HN can contribute.






Similar content being viewed by others
Abbreviations
- RDC:
-
residual dipolar coupling
References
Archer SJ, Ikura M, Torchia DA, Bax A (1991) An alternative 3D NMR technique for correlating backbone 15N with sidechain Hβ resonances in larger proteins. J Magn Reson 95:636–641
Bouvignies G, Bernado P, Meier S, Cho K, Grzesiek S, Bruschweiler R, Blackledge M (2005) Identification of slow correlated motions in proteins using residual dipolar and hydrogen-bond scalar couplings. Proc Natl Acad Sci U S A 102:13885–13890
Bouvignies G, Markwick P, Brueschweiler R, Blackledge M (2006) Simultaneous determination of protein backbone structure and dynamics from residual dipolar couplings. J Am Chem Soc 128:15100–15101
Bruschweiler R (2003) New approaches to the dynamic interpretation and prediction of NMR relaxation data from proteins. Curr Opin Struct Biol 13:175–183
Bystrov VF (1976) Spin–spin couplings and the conformational states of peptide systems. Prog. NMR Spectrosc 10:41–81
Cutting B, Tolman JR, Nanchen S, Bodenhausen G (2002) Accurate measurement of residual dipolar couplings in anisotropic phase. J Biomol NMR 23:195–200
de Alba E, Tjandra N (2006a) Interference between cross-correlated relaxation and the measurement of scalar and dipolar couplings by quantitative J. J Biomol NMR 35:1–16
de Alba E, Tjandra N (2006b) On the accurate measurement of amide one-bond N-15-H-1 couplings in proteins: effects of cross-correlated relaxation, selective pulses and dynamic frequency shifts. J Magn Reson 183:160–165
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRpipe–a multidimensional spectral processing system based on unix pipes. J Biomol NMR 6:277–293
Gayathri C, Bothnerby AA, Vanzijl PCM, Maclean C (1982) Dipolar Magnetic-Field effects in NMR spectra of liquids. Chem Phys Lett 87:192–196
Geen H, Freeman R (1991) Band-selective radiofrequency pulses. J Magn Reson 93:93–141
Goldman M (1984) Interference effects in the relaxation of a pair of unlike spin-1/2 nuclei. J Magn Reson 60:437–452
Griesinger C, Sorensen OW, Ernst RR (1985) Two-dimensional correlation of connected NMR transitions. J Am Chem Soc 107:6394–6396
Griesinger C, Sørensen OW, Ernst RR (1986) Correlation of connected transitions by two-dimensional NMR spectroscopy. J Chem Phys 85:6837–6852
Grzesiek S, Bax A (1993) The importance of not saturating H2O in protein NMR. Application to sensitivity enhancement and NOE measurement. J Am Chem Soc 115:12593–12594
Hall JB, Fushman D (2003) Characterization of the overall and local dynamics of a protein with intermediate rotational anisotropy: differentiating between conformational exchange and anisotropic diffusion in the B3 domain of protein G. J Biomol NMR 27:261–275
Hansen MR, Mueller L, Pardi A (1998) Tunable alignment of macromolecules by filamentous phage yields dipolar coupling interactions. Nat Struct Biol 5:1065–1074
Hus JC, Peti W, Griesinger C, Bruschweiler R (2003) Self-consistency analysis of dipolar couplings in multiple alignments of ubiquitin. J Am Chem Soc 125:5596–5597
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Lakomek NA, Carlomagno T, Becker S, Griesinger C, Meiler J (2006) A thorough dynamic interpretation of residual dipolar couplings in ubiquitin. J Biomol NMR 34:101–115
Lakomek NA, Walter KFA, Fares C, Lange OF, de Groot BL, Grubmuller H, Bruschweiler R, Munk A, Becker S, Meiler J, Griesinger C (2008) Self-consistent residual dipolar coupling based model-free analysis for the robust determination of nanosecond to microsecond protein dynamics. J Biomol NMR 41:139–155
Lange OF, Lakomek NA, Fares C, Schroder GF, Walter KFA, Becker S, Meiler J, Grubmuller H, Griesinger C, de Groot BL (2008) Recognition dynamics up to microseconds revealed from an RDC-derived ubiquitin ensemble in solution. Science 320:1471–1475
Meissner A, Schulte-Herbruggen T, Sorensen OW (1998) Spin-state-selective polarization or excitation for simultaneous E. COSY-type measurement of 3J(C′, Ha) coupling constants with enhanced sensitivity and resolution in multidimensional NMR spectroscopy of 13C, 15N– labeled proteins. Journal of American Chemical Society 120:3803–3804
Montelione GT, Wagner G (1989) Accurate measurements of homonuclear HN–Ha coupling constants in polypeptides using heteronuclear 2d NMR experiments. J Am Chem Soc 111:5474–5475
Ottiger M, Delaglio F, Bax A (1998a) Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J Magn Reson 131:373–378
Ottiger M, Delaglio F, Marquardt JL, Tjandra N, Bax A (1998b) Measurement of dipolar couplings for methylene and methyl sites in weakly oriented macromolecules and their use in structure determination. J Magn Reson 134:365–369
Peti W, Meiler J, Bruschweiler R, Griesinger C (2002) Model-free analysis of protein backbone motion from residual dipolar couplings. J Am Chem Soc 124:5822–5833
Prestegard JH, Al-Hashimi HM, Tolman JR (2000) NMR structures of biomolecules using field oriented media and residual dipolar couplings. Q Rev Biophys 33:371–424
Silver MS, Joseph RI, Hoult DI (1984) Highly selective Pi/2 and Pi-pulse generation. J Magn Reson 59:347–351
Tjandra N, Bax A (1997) Measurement of dipolar contributions to 1JCH splittings from magnetic-field dependence of J modulation in two-dimensional NMR spectra. J Magn Reson 124:512–515
Tjandra N, Grzesiek S, Bax A (1996) Magnetic field dependence of nitrogen-proton J splittings in N– 15– enriched human ubiquitin resulting from relaxation interference and residual dipolar coupling. J Am Chem Soc 118:6264–6272
Tjandra N, Omichinski JG, Gronenborn AM, Clore GM, Bax A (1997) Use of dipolar 1H–15N and 1H–13C couplings in the structure determination of magnetically oriented macromolecules in solution. Nat Struct Biol 4:732–738
Tolman JR (2002) A novel approach to the retrieval of structural and dynamic information from residual dipolar couplings using several oriented media in biomolecular NMR spectroscopy. J Am Chem Soc 124:12020–12030
Tolman JR, Ruan K (2006) NMR residual dipolar couplings as probes of biomolecular dynamics. Chem Rev 106:1720–1736
Tolman JR, Flanagan JM, Kennedy MA, Prestegard JH (1995) Nuclear Magnetic Dipole interactions in field-oriented proteins––information for structure determination in solution. Proc Natl Acad Sci U S A 92:9279–9283
Wagner G, Schmieder P, Thanabal V (1991) A new 1H–15N–13C triple resonance experiment for sequential assignments and measuring homonuclear Halpha-HN coupling constants in polypeptides. J Magn Reson 93:436–440
Wang AC, Bax A (1995) Reparametrization of the Karplus relation for (3)J(H-Alpha-N) and (3)J(H-N-C′) in peptides from uniformly C-13/N-15-enriched human ubiquitin. J Am Chem Soc 117:1810–1813
Wang AC, Bax A (1996) Determination of the backbone dihedral angles phi in human ubiquitin from reparametrized empirical Karplus equations. J Am Chem Soc 118:2483–2494
Werbelow LG (1996) The dynamic frequency shift. In: Grant DM, Harris RK (eds) Encyclopedia of nuclear magnetic resonance, vol 6. Wiley, London, pp 4072–4078
Yang DW, Nagayama K (1996) A sensitivity-enhanced method for measuring heteronuclear long-range coupling constants from the displacement of signals in two 1D subspectra. J Magn Reson Ser A 118:117–121
Yao LS, Bax A (2007) Modulating protein alignment in a liquid-crystalline medium through conservative mutagenesis. J Am Chem Soc 129:11326–11327
Yao L, Voegeli B, Ying JF, Bax A (2008a) NMR determination of amide N–H equilibrium bond length from concerted dipolar coupling measurements. J Am Chem Soc 130:16518–16520
Yao L, Vogeli B, Torchia DA, Bax A (2008b) Simultaneous NMR study of protein structure and dynamics using conservative mutagenesis. J Phys Chem B 112:6045–6056
Ying JF, Chill JH, Louis JM, Bax A (2007) Mixed-time parallel evolution in multiple quantum NMR experiments: sensitivity and resolution enhancement in heteronuclear NMR. J Biomol NMR 37:195–204
Zweckstetter M, Bax A (2002) Evaluation of uncertainty in alignment tensors obtained from dipolar couplings. J Biomol NMR 23:127–137
Acknowledgment
This work was supported in part by the Intramural Research Program of the NIDDK, NIH, and by the Intramural AIDS-Targeted Antiviral Program of the Office of the Director, NIH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yao, L., Ying, J. & Bax, A. Improved accuracy of 15N–1H scalar and residual dipolar couplings from gradient-enhanced IPAP-HSQC experiments on protonated proteins. J Biomol NMR 43, 161–170 (2009). https://doi.org/10.1007/s10858-009-9299-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-009-9299-x