Abstract
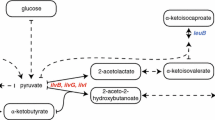
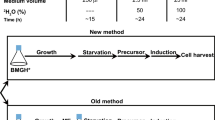
A strategy for the introduction of (1H,13C-methyl)-alanine into perdeuterated proteins is described. Specific protonation of alanine methyl groups to a level of 95% can be achieved by overexpressing proteins in M9/D2O based bacterial growth medium supplemented with 800 mg/l of 2-[2H], 3-[13C] l-alanine. However, though simple, this approach results in undesired, non-specific background labeling due to isotope scrambling via different amino acid metabolic pathways. Following a careful analysis of known metabolic pathways we found that co-addition of perdeuterated forms of α-ketoisovalerate-d7, succinate-d4 and l-isoleucine-d10 with labeled l-alanine, reduces undesired background labeling to <1%. When combined with recently developed methyl TROSY experiments, this methyl-specific labeling protocol permits the acquisition of excellent quality correlation spectra of alanine methyl groups in high molecular weight proteins. Our cost effective strategy offers a significant enhancement in the level of incorporation of methyl-labeled alanine in overexpressed proteins over previously reported methods.





Similar content being viewed by others
References
Arora A, Abildgaard F, Bushweller JH, Tamm LK (2001) Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nat Struct Biol 8:334–338
Brutscher B, Boisbouvier J, Pardi A, Marion D, Simorre JP (1998) Improved sensitivity and resolution in 1H-13C NMR experiments of RNA. J Am Chem Soc 120:11845–11851
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Fernandez C, Adeishvili K, Wüthrich K (2001) Transverse relaxation-optimized NMR spectroscopy with the outer membrane protein OmpX in dihexanoyl phosphatidylcholine micelles. Proc Natl Acad Sci USA 98:2358–2363
Fiaux J, Bertelsen EB, Horwich AL, Wüthrich K (2002) NMR analysis of a 900 kDa GroEL GroES complex. Nature 418:207–211
Gardner KH, Kay LE (1997) Production and incorporation of 15N, 13C, 2H (1H–δ1 Methyl) isoleucine into proteins for multidimensional NMR studies. J Am Chem Soc 119:7599–7600
Gardner KH, Kay LE (1998) The use of 2H, 13C, 15N multidimensional NMR to study the structure and dynamics of proteins. Ann Rev Biophys Biomol Struct 27:357–406
Goss RJM, Newill PLA (2006) A convenient enzymatic synthesis of l-halotryptophans. Chem Commun 4924–4925
Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE (1999) A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR 13:369–374
Hamel DJ, Dahlquist FW (2005) The contact interface of a 120 kD CheA-CheW complex by methyl TROSY interaction spectroscopy. J Am Chem Soc 127:9676–9677
Isaacson RL, Simpson PJ, Liu M, Cota E, Zhang X, Freemont P, Matthews S (2007) A new labelling method for methyl transverse relaxation-optimized spectroscopy NMR spectra of Alanine residues. J Am Chem Soc 129:15428–15429
McCaldon P, Argos P (1988) Oligopeptide biases in protein sequences and their use in predicting protein coding regions in nucleotide-sequences. Proteins Struct Funct Genet 4:99–122
Miclet E, Williams JDC, Clore GM, Bryce DL, Boisbouvier J, Bax A (2004) Relaxation-optimized NMR spectroscopy of methylene groups in proteins and nucleic acids. J Am Chem Soc 126:10560–10570
Miclet E, Boisbouvier J, Bax A (2005) Measurement of eight scalar and dipolar couplings for methine-methylene pairs in proteins and nucleic acids. J Biomol NMR 31:201–216
Muchmore DC, McIntosh LP, Russel CB, Anderson DE, Dahlquist FW (1989) Expression and 15N labelling of proteins for proton and 15N nuclear magnetic resonance. Meth Enzymol 177:44–73
Nibeilliu ME, Malthouse PG (2004) The stereospecificity and catalytic efficiency of the tryptophan synthase-catalysed exchange of the α-protons of amino acids. Biochem J 381:847–852
Pervushin K, Riek R, Wider G, Wuthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole- dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA 94:12366–12371
Pervushin K, Riek R, Wider G, Wuthrich K (1998) Transverse relaxation-optimized spectroscopy (TROSY) for NMR studies of aromatic spin systems in C-13-labeled proteins. J Am Chem Soc 120:6394–6400
Riek R, Wider G, Pervushin K, Wüthrich K (1999) Polarization transfer by cross-correlated relaxation in solution NMR with very large molecules. Proc Natl Acad Sci USA 96:4918–4923
Rosen MK, Gardner KH, Willis RC, Parris WE, Pawson T, Kay LE (1996) Selective methyl group protonation of perdeuterated proteins. J Mol Biol 263:627–636
Salzmann M, Pervushin K, Wider G, Senn H, Wüthrich K (2000) NMR assignment and secondary structure determination of an octameric 110 kDa protein using TROSY in triple resonance experiments. J Am Chem Soc 122:7543–7548
Sounier R, Blanchard L, Wu Z, Boisbouvier J (2007) High-accuracy distance measurement between remote methyls in specifically protonated proteins. J Am Chem Soc 129:472–473
Sprangers R, Kay LE (2007) Quantitative dynamics and binding studies of the 20S proteasome by NMR. Nature 445:618–622
Sprangers R, Gribun A, Hwang PM, Houry WA, Kay LE (2005) Quantitative NMR spectroscopy of supramolecular complexes: Dynamics side pores in ClpP are important for product release. Proc Natl Acad Sci USA 102:16678–16683
Sprangers R, Velyvis A, Kay LE (2007) Solution NMR of supramolecular complexes: providing new insights into function. Nat Meth 4:697–703
Tugarinov V, Kay LE (2004) An isotope labeling strategy for methyl TROSY spectroscopy. J Biomol NMR 28:165–172
Tugarinov V, Muhandiram DR, Ayed A, Kay LE (2002) Four-dimensional NMR spectroscopy of a 723-residue protein: chemical shift assignments and secondary structure of malate synthase G. J Am Chem Soc 124:10025–10035
Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE (2003) Cross-correlated relaxation enhanced 1H–13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc 125:10420–10428
Tugarinov V, Choy WY, Orekhov VY, Kay LE (2005) Solution NMR-derived global folded of a monomeric 82-kDA enzyme. Proc Natl Acad Sci USA 102:622–627
Tugarinov V, Kanelis V, Kay LE (2006) Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nat Protoc 1:749–754
Vanatalu K, Paalme T, Vilu R, Burkhardt N, Junemann R, May R, Ruhl M, Wadzack J, Nierhaus KH (1993) Large-scale preparation of fully deuterated cell components—ribosomes from Escherichia coli with high biological activity. Eur J Biochem 216:315–321
Venters RA, Huang C-C, Farmer BTII, Trolard R, Spicer LD, Fierke CA (1995) High-level 2H/13C/15N labeling of proteins for NMR studies. J Biomol NMR 5:339–344
Acknowledgements
This work was supported by the CEA, the CNRS, and the UJF. J.B acknowledges funding from HFSP (CDA #0029/2004) and CNRS (Interdisciplinary program interface between physics, biology and chemistry). The authors thank Drs. Kay and Remington for providing clones of Malate Synthase G, Drs. Amero and Marion for stimulating discussion, and Dr. Plevin for suggestions and careful reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ayala, I., Sounier, R., Usé, N. et al. An efficient protocol for the complete incorporation of methyl-protonated alanine in perdeuterated protein. J Biomol NMR 43, 111–119 (2009). https://doi.org/10.1007/s10858-008-9294-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-008-9294-7