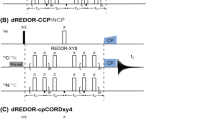
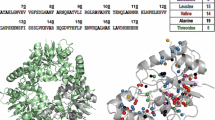
Abstract
Cross-saturation experiments allow the identification of the contact residues of large protein complexes (MW>50 K) more rigorously than conventional NMR approaches which involve chemical shift perturbations and hydrogen-deuterium exchange experiments [Takahashi et al. (2000) Nat. Struct. Biol., 7, 220–223]. In the amide proton-based cross-saturation experiment, the combined use of high deuteration levels for non-exchangeable protons of the ligand protein and a solvent with a low concentration of 1H2O greatly enhanced the selectivity of the intermolecular cross-saturation phenomenon. Unfortunately, experimental limitations caused losses in sensitivity. Furthermore, since main chain amide protons are not generally exposed to solvent, the efficiency of the saturation transfer directed to the main chain amide protons is not very high. Here we propose an alternative cross-saturation experiment which utilizes the methyl protons of the side chains of the ligand protein. Owing to the fast internal rotation along the methyl axis, we theoretically and experimentally demonstrated the enhanced efficiency of this approach. The methyl-utilizing cross-saturation experiment has clear advantages in sensitivity and saturation transfer efficiency over the amide proton-based approach.
Similar content being viewed by others
References
F. Delaglio S. Grzesiek G.W. Vuister G. Zhu J. Pfeifer A. Bax (1995) J. Biomol. NMR 6 277–293 Occurrence Handle10.1007/BF00197809
E.Z. Eisenmesser A.P.R. Zabell C.B. Post (2000) J. Biomol. NMR 17 17–32 Occurrence Handle10.1023/A:1008311703619
Y. Fukunishi Y. Mikami H. Nakamura (2003) J. Phys. Chem. B. 103 13201–13210
N.K. Goto K.H. Gardner G.A. Mueller R.C. Willis L.E. Kay (1999) J. Biomol. NMR 13 369–374 Occurrence Handle10.1023/A:1008393201236
H. Gouda H. Torigoe A. Saito M. Sato Y. Arata I. Shimada (1992) Biochemistry 31 9665–9672 Occurrence Handle10.1021/bi00155a020
P.J. Hajduk D.J. Augeri J. Mack R. Mendoza J.G. Yang S.F. Betz S.W. Fesik (2000) J. Am. Chem. Soc. 122 7898–7904 Occurrence Handle10.1021/ja000350l
D.J. Hamel F.W. Dahlquist (2005) J. Am. Chem. Soc. 127 9676–9677 Occurrence Handle10.1021/ja052517m
C. Hilty C. Fernandez G. Wider K. Wüthrich (2002) J. Biomol. NMR 23 289–301 Occurrence Handle10.1023/A:1020218419190
Z. Hu B. Ma H. Wolfson R. Nussinov (2000) Proteins 39 331–342 Occurrence Handle10.1002/(SICI)1097-0134(20000601)39:4<331::AID-PROT60>3.0.CO;2-A
R. Ishima S. Shibata K. Akasaka (1991) J. Magn. Reson. 91 455–465
V. Jayalakshmi N.R. Krishna (2002) J. Magn. Reson. 155 106–118 Occurrence Handle2002JMagR.155..106J Occurrence Handle10.1006/jmre.2001.2499
R. Koradi M. Billeter K. Wüthrich (1996) J. Mol. Graphics 14 29–32
E. Kupce G. Wagner (1995) J. Magn. Reson. B 109 329–333
A.N. Lane G. Kelly A. Ramos T.A. Frenkiel (2001) J. Biomol. NMR 21 127–139 Occurrence Handle10.1023/A:1012486527215
L. Lo Conte C. Chothia J. Janin (1999) J. Mol. Biol. 285 2177–2198
F. Lohr H. Ruterjans (2002) J. Magn. Reson. 156 10–18 Occurrence Handle2002JMagR.156...10L Occurrence Handle10.1006/jmre.2002.2539
B. Ma T. Elkayam H. Wolfson R. Nussinov (2003) Proc. Natl. Acad. Sci. USA 100 5772–5777 Occurrence Handle2003PNAS..100.5772M
L. Müller (1979) J. Am. Chem. Soc. 101 4481–4484
T. Nakanishi M. Miyazawa M. Sakakura H. Terasawa H. Takahashi I. Shimada (2002) J. Mol. Biol. 318 245–249 Occurrence Handle10.1016/S0022-2836(02)00018-9
J.E. Ollerenshaw V. Tugarinov L.E. Kay (2003) Magn. Reson. Chem. 41 843–852 Occurrence Handle10.1002/mrc.1256
A. Ross M. Salzmann H. Senn (1997) J. Biomol. NMR 10 389–396 Occurrence Handle10.1023/A:1018361214472
H. Senn B. Werner B.A. Messerle C. Weber R. Traber K. Wüthrich (1989) FEBS Lett. 249 113–118 Occurrence Handle10.1016/0014-5793(89)80027-4
H. Takahashi T. Nakanishi K. Kami Y. Arata I. Shimada (2000) Nat. Struct. Biol. 7 220–223
H. Torigoe I. Shimada A. Saito M. Sato Y. Arata (1990) Biochemistry 29 8787–8793 Occurrence Handle10.1021/bi00489a040
V. Tugarinov P.M. Hwang J.E. Ollerenshaw L.E. Kay (2003) J. Am. Chem. Soc. 125 10420–10428
V. Tugarinov L.E. Kay (2003a) J. Am. Chem. Soc. 125 5701–5706
V. Tugarinov L.E. Kay (2003b) J. Am. Chem. Soc. 125 13868–13878
G.W. Vuister A. Bax (1992) J. Magn. Reson. 98 428–435
G. Wider D. Neri K. Wüthrich (1991) J. Biomol. NMR 1 93–98 Occurrence Handle10.1007/BF01874572
D.W. Yang Y. Zheng D.J. Liu D.F. Wyss (2004) J. Am. Chem. Soc. 126 3710–3711
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Takahashi, H., Miyazawa, M., Ina, Y. et al. Utilization of Methyl Proton Resonances in Cross-Saturation Measurement for Determining the Interfaces of Large Protein–Protein Complexes. J Biomol NMR 34, 167–177 (2006). https://doi.org/10.1007/s10858-006-0008-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10858-006-0008-8