Abstract
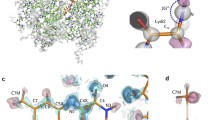
Acireductone dioxygenase (ARD) from Klebsiella ATCC 8724 is a metalloenzyme that is capable of catalyzing different reactions with the same substrates (acireductone and O2) depending upon the metal bound in the active site. A model for the solution structure of the paramagnetic Ni2+-containing ARD has been refined using residual dipolar couplings (RDCs) measured in two media. Additional dihedral restraints based on chemical shift (TALOS) were included in the refinement, and backbone structure in the vicinity of the active site was modeled from a crystallographic structure of the mouse homolog of ARD. The incorporation of residual dipolar couplings into the structural refinement alters the relative orientations of several structural features significantly, and improves local secondary structure determination. Comparisons between the solution structures obtained with and without RDCs are made, and structural similarities and differences between mouse and bacterial enzymes are described. Finally, the biological significance of these differences is considered.
Similar content being viewed by others
References
F. Al-Mjeni T. Ju T.C. Pochapsky M.J. Maroney (2002) Biochemistry 41 6761–6769 Occurrence Handle10.1021/bi012209a
R. Anand P.C. Dorrestein C. Kinsland T.P. Begley S.E. Ealick (2002) Biochemistry 41 7659–7669
C. Bartels T.H. Xia M. Billeter P. Guntert K. Wuthrich (1995) J. Biomol. NMR 6 1–10 Occurrence Handle10.1007/BF00417486
G. Cornilescu F. Delaglio A. Bax (1999) J. Biomol. NMR 13 289–302 Occurrence Handle10.1023/A:1008392405740
Y. Dai P.C. Wensink R.H. Abeles (1999) J. Biol. Chem 274 1193–1195
Y. Dai T.C. Pochapsky R.H. Abeles (2001) Biochemistry 40 6379–6387 Occurrence Handle10.1021/bi010110y
J.M. Dunwell A. Culham C.E. Carter C.R. Sosa-Aguirre P.W. Goodenough (2001) Trends Biochem.Sci. 26 740–746 Occurrence Handle10.1016/S0968-0004(01)01981-8
M.R. Hansen L. Mueller A. Pardi (1998) Nature Struct. Biol. 5 1065–1074
R.W.W. Hooft G. Vriend C. Sander E.E. Abola (1996) Nature 381 272 Occurrence Handle10.1038/381272a0 Occurrence Handle1996Natur.381..272H
JCSG, the Joint Center for Structural Genomics (2005) Protein Data Base Entry 1VR3
P. Kestell (1996) Charged sulfur compounds S. Mitchell (Eds) Biological Interactions of Sulfur Compounds Taylor & Francis London 180–221
T.P. Ko J. Day A. Mcpherson (2000) Acta Crystall. Sec. D – Biol. Crystallogr. 56 411–420
R. Koradi M. Billeter K. Wuthrich (1996) J. Mol. Graphics 14 51
M. Kostic S.S. Pochapsky T.C. Pochapsky (2002) J. Am. Chem. Soc. 124 9054–9055 Occurrence Handle10.1021/ja0268480
P.J. Kraulis (1991) J. Appl. Crystallogr. 24 946–950 Occurrence Handle10.1107/S0021889891004399
J. Kuszewski A.M. Gronenborn G.M. Clore (1996) Prot. Sci. 5 1067–1080
J. Kuszewski A.M. Gronenborn G.M. Clore (1997) J. Magn. Reson. 125 171–177 Occurrence Handle10.1006/jmre.1997.1116
J. Kuszewski G.M. Clore (2000) J. Magn. Reson. 146 249–254 Occurrence Handle10.1006/jmre.2000.2142 Occurrence Handle2000JMagR.146..249K
R.A. Laskowski J.A.C. Rullmann M.W. Macarthur R. Kaptein J.M. Thornton (1996) J. Biomol. NMR 8 477–486 Occurrence Handle10.1007/BF00228148
H.P. Mo Y. Dai S.S. Pochapsky T.C. Pochapsky (1999) J. Biomol. NMR 14 287–288 Occurrence Handle10.1023/A:1008396624784
D.R. Muhandiram L.E. Kay (1994) J. Magn. Reson. Series B 103 203–216
T.C. Pochapsky S.S. Pochapsky T.T. Ju H.P. Mo F. Al-Mjeni M.J. Maroney (2002) Nature. Struct. Biol. 9 966–972 Occurrence Handle10.1038/nsb863
J.H. Prestegard C.M. Bougault A.I. Kishore (2004) Chem. Rev. 104 3519–3540 Occurrence Handle10.1021/cr030419i
M. Ruckert G. Otting (2000) J. Am. Chem. Soc. 122 7793–7797 Occurrence Handle10.1021/ja001068h
E. Szajna P. Dobrowolski A.L. Fuller A.M. Arif L.M. Berreau (2004) Inorg. Chem. 43 3988–3997 Occurrence Handle10.1021/ic040002a
N. Tjandra J.G. Omichinski A.M. Gronenborn G.M. Clore A. Bax (1997) Nature Struct. Biol. 4 732–738
E.J. Woo J.M. Dunwell P.W. Goodenough A.C. Marvier R.W. Pickersgill (2000) Nature Struct. Biol. 7 1036–1040
J.W. Wray R.H. Abeles (1993) J. Biol .Chem. 268 21466–21469
M. Zweckstetter A. Bax (2000) J. Am. Chem. Soc. 122 3791–3792 Occurrence Handle10.1021/ja0000908
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pochapsky, T.C., Pochapsky, S.S., Ju, T. et al. A Refined Model for the Structure of Acireductone Dioxygenase from Klebsiella ATCC 8724 Incorporating Residual Dipolar Couplings. J Biomol NMR 34, 117–127 (2006). https://doi.org/10.1007/s10858-005-5735-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10858-005-5735-8