Abstract
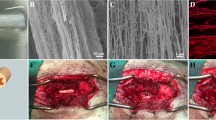
Spinal cord injuries (SCI) normally disrupt the long axonal tracts of the spinal cord and cause permanent neurological deficits, for which there is currently a lack of effective therapeutic methods. Biomaterial-based regenerative medicine is a pivotal strategy to induce axonal regeneration through delivery of biophysical and/or biochemical regulatory cues by biomaterials. We previously fabricated a hierarchically aligned fibrin hydrogel (AFG) that could promote neurogenic differentiation of stem cells in vitro and has been successfully applied for peripheral nerve and spinal cord regeneration in rats. In this study, AFG was used to repair a canine lumbar segment 2 hemisection spinal cord injury, and the consistency of histological, imageological and behavioral results was compared. AFG was used to construct an aligned fiber bridge that supported cell adhesion in vitro and rapidly facilitated tissue invasion along the long axis of fibers in vivo, Moreover, in vivo results demonstrated regrowth of axons in an oriented pattern connecting the rostral and caudal stumps. Consistent results were confirmed by diffusion tensor imaging, which allowed successful tracing of reconnected nerve fibers across the defect. As a result, directional axonal regrowth contributed to significantly improved recovery of motor functional behavior of SCI canines with AFG implantation. Our results suggest that AFG has great promise for rapidly directing axonal regrowth for nerve regeneration.











Similar content being viewed by others
References
Kabu S, Gao Y, Kwon BK, Labhasetwar V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J Control Release. 2015;219:141–54. https://doi.org/10.1016/j.jconrel.2015.08.060.
Han S, Wang B, Jin W, Xiao Z, Li X, Ding W, et al. The linear-ordered collagen scaffold-BDNF complex significantly promotes functional recovery after completely transected spinal cord injury in canine. Biomaterials. 2015;41:89–96. https://doi.org/10.1016/j.biomaterials.2014.11.031.
Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637. https://doi.org/10.1038/nn.4541.
Li G, Che M-T, Zhang K, Qin L-N, Zhang Y-T, Chen R-Q, et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233–48. https://doi.org/10.1016/j.biomaterials.2015.11.059.
Yang Z, Zhang A, Duan H, Zhang S, Hao P, Ye K, et al. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci USA. 2015;112:13354–9. https://doi.org/10.1073/pnas.1510194112.
Milbreta U, Nguyen LH, Diao H, Lin J, Wu W, Sun C-Y, et al. Three-dimensional nanofiber hybrid scaffold directs and enhances axonal regeneration after spinal cord injury. ACS Biomater Sci Eng. 2016;2:1319–29. https://doi.org/10.1021/acsbiomaterials.6b00248.
Gao M, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto J, et al. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials. 2013;34:1529–36. https://doi.org/10.1016/j.biomaterials.2012.10.07.
Koffler J, Samara RF, Rosenzweig ES. Using templated agarose scaffolds to promote axon regeneration through sites of spinal cord injury. Axon growth and regeneration: methods and protocols. 2014:157–65. https://doi.org/10.1007/978-1-4939-0777-9.
Guenther MI, Weidner N, Müller R, Blesch A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 2015;27:140–50. https://doi.org/10.1016/j.actbio.2015.09.001.
Berns EJ, Sur S, Pan L, Goldberger JE, Suresh S, Zhang S, et al. Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials. 2014;35:185–95. https://doi.org/10.1016/j.biomaterials.2013.09.07.
Thomas AM, Kubilius MB, Holland SJ, Seidlits SK, Boehler RM, Anderson AJ, et al. Channel density and porosity of degradable bridging scaffolds on axon growth after spinal injury. Biomaterials. 2013;34:2213–20. https://doi.org/10.1016/j.biomaterials.2012.12.002.
Yang Y, Laporte LD, Zelivyanskaya ML, Whittlesey KJ, Anderson AJ, Cummings BJ, et al. Multiple channel bridges for spinal cord injury: cellular characterization of host response. Tissue Eng A. 2009;15:3283–95. https://doi.org/10.1089/ten.tea.2009.0081.
Li A, Hokugo A, Yalom A, Berns EJ, Stephanopoulos N, McClendon MT, et al. A bioengineered peripheral nerve construct using aligned peptide amphiphile nanofibers. Biomaterials. 2014;35:8780–90. https://doi.org/10.1016/j.biomaterials.2014.06.04.
Greene JJ, McClendon MT, Stephanopoulos N, Álvarez Z, Stupp SI, Richter CP. Electrophysiological assessment of a peptide amphiphile nanofiber nerve graft for facial nerve repair. J Tissue Eng Regen Med. 2018;12:1389–401. https://doi.org/10.1002/term.2669.
Tibbitt MW, Rodell CB, Burdick JA, Anseth KS. Progress in material design for biomedical applications. Proc Natl Acad Sci. 2015;112:14444–51. https://doi.org/10.1073/pnas.1516247112.
Assunção-Silva RC, Gomes ED, Sousa N, Silva NA, Salgado AJ. Hydrogels and cell based therapies in spinal cord injury regeneration. Stem Cells Int. 2015;2015. https://doi.org/10.1155/2015/948040.
Kubinová Š, Horák D, Hejčl A, Plichta Z, Kotek J, Proks V, et al. SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores for spinal cord injury repair. J Tissue Eng Regen Med. 2015;9:1298–309. https://doi.org/10.1002/term.1694.
Yao S, Liu X, Yu S, Wang X, Zhang S, Wu Q, et al. Co-effects of matrix low elasticity and aligned topography on stem cell neurogenic differentiation and rapid neurite outgrowth. Nanoscale. 2016;8:10252–65. https://doi.org/10.1039/c6nr01169a.
Zhang Z, Yao S, Xie S, Wang X, Chang F, Luo J, et al. Effect of hierarchically aligned fibrin hydrogel in regeneration of spinal cord injury demonstrated by tractography: a pilot study. Sci Rep. 2017;7:40017. https://doi.org/10.1038/srep40017.
Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. https://doi.org/10.1126/science.1191035.
Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–8. https://doi.org/10.1529/biophysj.105.073114.
Han S, Wang B, Jin W, Xiao Z, Chen B, Xiao H, et al. The collagen scaffold with collagen binding BDNF enhances functional recovery by facilitating peripheral nerve infiltrating and ingrowth in canine complete spinal cord transection. Spinal Cord. 2014;52:867–73. https://doi.org/10.1038/sc.2014.173.
Fan C, Li X, Xiao Z, Zhao Y, Liang H, Wang B, et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51:304–16. https://doi.org/10.1016/j.actbio.2017.01.009.
Liu Y, Ye H, Satkunendrarajah K, et al. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013;9:8075–88. https://doi.org/10.1016/j.actbio.2013.06.001.
Slotkin JR, Pritchard CD, Luque B, et al. Biodegradable scaffolds promote tissue remodeling and functional improvement in non-human primates with acute spinal cord injury. Biomaterials. 2017;123:63–76. https://doi.org/10.1016/j.biomaterials.2017.01.024.
Tsintou M, Dalamagkas K, Seifalian AM. Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res. 2015;10:726. https://doi.org/10.4103/1673-5374.156966.
Yao S, Yu S, Cao Z, et al. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int J Nanomed. 2018;13:2883–95. https://doi.org/10.2147/ijn.s159356.
Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–45. https://doi.org/10.1016/j.biomaterials.2010.06.061.
Yang F, Wang J, Cao L, et al. Injectable and redox-responsive hydrogel with adaptive degradation rate for bone regeneration. J Mat Chem B. 2014;2:295–304. https://doi.org/10.1039/c3tb21103g.
Sacchi V, Mittermayr R, Hartinger J, et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF(164). Proc Natl Acad Sci USA. 2014;111:6952–7. https://doi.org/10.1073/pnas.1404605111.
Funding
This work was supported by funds provided by National Natural Science Foundation of China (No. 31771056), National Key R&D Program of China (2018YFB0704304-1) and Tsinghua University Initiative Scientific Research Program (20161080091).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cao, Z., Yao, S., Xiong, Y. et al. Directional axonal regrowth induced by an aligned fibrin nanofiber hydrogel contributes to improved motor function recovery in canine L2 spinal cord injury. J Mater Sci: Mater Med 31, 40 (2020). https://doi.org/10.1007/s10856-020-06375-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06375-9