Abstract
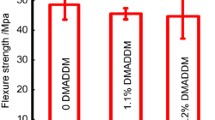
The objectives of this study were to: (1) develop a new bioactive dental bonding agent with nanoparticles of amorphous calcium phosphate and dimethylaminohexadecyl methacrylate for tooth root caries restorations and endodontic applications, and (2) investigate biofilm inhibition by the bioactive bonding agent against eight species of periodontal and endodontic pathogens for the first time. Bonding agent was formulated with 5 % of dimethylaminohexadecyl methacrylate. Nanoparticles of amorphous calcium phosphate at 30 wt% was mixed into adhesive. Eight species of biofilms were grown on resins: Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Parvimonas micra, Enterococcus faecalis, Enterococcus faecium. Colony-forming units, live/dead assay, biomass, metabolic activity and polysaccharide of biofilms were determined. The results showed that adding dimethylaminohexadecyl methacrylate and nanoparticles of amorphous calcium phosphate into bonding agent did not decrease dentin bond strength (P > 0.1). Adding dimethylaminohexadecyl methacrylate reduced the colony-forming units of all eight species of biofilms by nearly three orders of magnitude. The killing efficacy of dimethylaminohexadecyl methacrylate resin was: P. gingivalis > A. actinomycetemcomitans > P. intermedia > P. nigrescens > F. nucleatum > P. micra > E. faecalis > E. faecium. Dimethylaminohexadecyl methacrylate resin had much less biomass, metabolic activity and polysaccharide of biofilms than those without dimethylaminohexadecyl methacrylate (P < 0.05). In conclusion, a novel dental adhesive was developed for root caries and endodontic applications, showing potent inhibition of biofilms of eight species of periodontal and endodontic pathogens, and reducing colony-forming units by three orders of magnitude. The bioactive adhesive is promising for tooth root restorations to provide subgingival margins with anti-periodontal pathogen capabilities, and for endodontic sealer applications to combat endodontic biofilms.






Similar content being viewed by others
References
Griffin S, Griffin P, Swann J, Zlobin N. Estimating rates of new root caries in older adults. J Dent Res. 2004;83:634–8.
Shen S, Samaranayake LP, Yip HK. In vitro growth, acidogenicity and cariogenicity of predominant human root caries flora. J Dent. 2004;32:667–78.
Hellyer P, Beighton D, Heath M, Lynch E. Root caries in older people attending a general dental practice in east sussex. Brit Dent J. 1990;169:201–6.
Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent. 2007;35:201–6.
Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of eight putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266–73.
Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16s clonal analysis. J Clin Microbiol. 2005;43:3944–55.
Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J Dent Res. 2006;85:392–403.
Fteita D, Könönen E, Söderling E, Gürsoy UK. Effect of estradiol on planktonic growth, coaggregation, and biofilm formation of the prevotella intermedia group bacteria. Anaerobe. 2014;27:7–13.
Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, et al. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol. 2007;45:3859–69.
Gürsoy M, Haraldsson G, Hyvönen M, Sorsa T, Pajukanta R, Könönen E. Does the frequency of prevotella intermedia increase during pregnancy? Oral Microbiol Immunol. 2009;24:299–303.
Signat B, Roques C, Poulet P, Duffaut D. Role of fusobacterium nucleatum in periodontal health and disease. Curr Issues Mol Biol. 2011;13:25–36.
Shchipkova A, Nagaraja H, Kumar P. Subgingival microbial profiles of smokers with periodontitis. J Dent Res. 2010;89:1247–53.
Saleh I, Ruyter I, Haapasalo M, Ørstavik D. Survival of enterococcus faecalis in infected dentinal tubules after root canal filling with different root canal sealers in vitro. Int Endod J. 2004;37:193–8.
Ferrari P, Cai S, Bombana A. Effect of endodontic procedures on enterococci, enteric bacteria and yeasts in primary endodontic infections. Int Endod J. 2005;38:372–80.
Ferracane JL. Resin composite-state of the art. Dent Mater. 2011;27:29–38.
Wei YJ, Silikas N, Zhang ZT, Watts DC. Hygroscopic dimensional changes of self-adhering and new resin-matrix composites during water sorption/desorption cycles. Dent Mater. 2011;27:259–66.
Pashley DH, Tay FR. Aggressiveness of contemporary self-etching adhesives: Part ii: etching effects on unground enamel. Dent Mater. 2001;17:430–44.
Ferracane JL, Hilton TJ, Sakaguchi RL. Introduction to and outcomes of the conference on adhesion in dentistry. Dent Mater. 2010;26:105–7.
Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing mdpb after curing. J Dent. 1998;26:267–71.
Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–57.
Li F, Weir MD, Xu HH. Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res. 2013;92:932–8.
Zhang L, Weir MD, Hack G, Fouad AF, Xu HH. Rechargeable dental adhesive with calcium phosphate nanoparticles for long-term ion release. J Dent. 2015;43:1587–95.
Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J Dent Res. 2012;91:979–84.
Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–9.
Li F, Weir MD, Chen J, Xu HH. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent Mater. 2014;30:433–41.
Antonucci JM, O’Donnell JNR, Schumacher GE, Skrtic D. Amorphous calcium phosphate composites and their effect on composite–adhesive–dentin bonding. J Adhes Sci Technol. 2009;23:1133–47.
Nadkarni MA, Martin FE, Hunter N, Jacques NA. Methods for optimizing DNA extraction before quantifying oral bacterial numbers by real-time PCR. FEMS Microbiol Lett. 2009;296:45–51.
Chen C, Weir MD, Cheng L, Lin NJ, Lin-Gibson S, Chow L, et al. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent Mater. 2014;30:891–901.
Ebi N, Imazato S, Noiri Y, Ebisu S. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent Mater. 2001;17:485–91.
Masdea L, Kulik E, Hauser-Gerspach I, Ramseier A, Filippi A, Waltimo T. Antimicrobial activity of streptococcus salivarius k12 on bacteria involved in oral malodour. Arch Oral Biol. 2012;57:1041–7.
Pitts B, Hamilton MA, Zelver N, Stewart PS. A microtiter-plate screening method for biofilm disinfection and removal. J Microbiol Methods. 2003;54:269–76.
Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on prevalence of periodontitis in adults in the united states: Nhanes 2009 to 2012. J Periodontol. 2015;86:611–22.
Kim JK, Baker LA, Seirawan H, Crimmins EM. Prevalence of oral health problems in us adults, nhanes 1999–2004: exploring differences by age, education, and race/ethnicity. Spec Care Dent. 2012;32:234–41.
National Toxicology Program. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. National Toxicology Program Center for the Evaluation of Risks to Human Reproduction. 2008.
Skrtic D, Antonucci JM, Eanes ED. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J Res Natl Inst Stand Technol. 2003;108:167–82.
Chen L, Suh BI. Bisphenol A in dental materials: a review. JSM Dent. 2013;1:1004.
Karapinar-Kazandag M, Bayrak OF, Yalvaç ME, Erev H, Tanalp J, Sahin F, et al. Cytotoxicity of 5 endodontic sealers on L929 cell line and human dental pulp cells. Int Endod J. 2011;44:626–34.
Xu P, Liang J, Dong G, Zheng L, Ye L. Cytotoxicity of RealSeal on human osteoblast-like MG63 cells. J Endod. 2010;36:40–4.
Onay EO, Ungor M, Ozdemir BH. In vivo evaluation of the biocompatibility of a new resin-based obturation system. Oral Surg Oral Med Oral Pathol Oral Radiol. 2007;104:60–6.
Garcia Lda F, Marques AA, Roselino Lde M, Pires-de-Souza Fde C, Consani S. Biocompatibility evaluation of Epiphany/Resilon root canal filling system in subcutaneous tissue of rats. J Endod. 2010;36:110–4.
Cotton TP, Schindler WG, Schwartz SA, Watson WR, Hargreaves KM. A retrospective study comparing clinical outcomes after obturation with Resilon/Epiphany or Gutta-Percha/Kerr sealer. J Endod. 2008;34:789–97.
Palmer L, Chapple I, Wright H, Roberts A, Cooper P. Extracellular deoxyribonuclease production by periodontal bacteria. J Periodontal Res. 2012;47:439–45.
Spratt D, Pratten J, Wilson M, Gulabivala K. An in vitro evaluation of the antimicrobial efficacy of irrigants on biofilms of root canal isolates. Int Endod J. 2001;34:300–7.
Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–33.
Daoud NN, Dickinson NA, Gilbert P. Antimicrobial activity and physico-chemical properties of some alkyldimethylbenzylammonium chlorides. Microbios. 1982;37:73–85.
Gilbert P, Al-taae A. Antimicrobial activity of some alkyltrimethylammonium bromides. Lett Appl Microbiol. 1985;1:101–4.
Backlund CJ, Sergesketter A, Offenbacher S, Schoenfisch M. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J Dent Res. 2014;93:1089–94.
Vargas-Reus MA, Memarzadeh K, Huang J, Ren GG, Allaker RP. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int J Antimicrob Agents. 2012;40:135–9.
Hosaka Y, Saito A, Maeda R, Fukaya C, Morikawa S, Makino A, et al. Antibacterial activity of povidone–iodine against an artificial biofilm of porphyromonas gingivalis and fusobacterium nucleatum. Arch Oral Biol. 2012;57:364–68.
Mundy L, Sahm D, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev. 2000;13:513–22.
Rams TE, Feik D, Mortensen JE, Degener JE, van Winkelhoff AJ. Antibiotic susceptibility of periodontal enterococcus faecalis. J Periodontol. 2013;84:1026–33.
Kitagawa R, Kitagawa H, Izutani N, Hirose N, Hayashi M, Imazato S. Development of an antibacterial root canal filling system containing mdpb. J Dent Res. 2014;93:1277–82.
Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against streptococcus mutans. Biomaterials. 2006;27:3995–4002.
Stepanović S, Vuković D, Dakić I, Savić B, Švabić-Vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175–79.
Pöllänen MT, Paino A, Ihalin R. Environmental stimuli shape biofilm formation and the virulence of periodontal pathogens. Int J Mol Sci. 2013;14:17221–37.
Rimondini L, Palazzo B, Iafisco M, Canegallo L, Demarosi F, Merlo M, et al. The remineralizing effect of carbonate-hydroxyapatite nanocrystals on dentine. Mater Sci Forum. 2007;539:602–5. Trans Tech Publications.
Besinis A, van Noort R, Martin N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent Mater. 2012;28:1012–23.
Li F, Wang P, Weir MD, Fouad AF, Xu HHK. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 2014;10:2804–13.
Acknowledgments
This work was supported by NIH R01DE17974 (HX), National Science Foundation of China NSFC 81400487 (LW), 81200820 (XX), Youth Fund of Science and Technology Jilin Province 20150520043JH (LW), China Postdoctoral Foundation 2015M581405 (LW), China Scholarship Council (LW), NSFC 31328008 (LZ), NSF Guangdong 20130010014253 (LZ) and 2014A030313275 (LZ), Guangdong Science and Technology 2012B010200024 (LZ), and University of Maryland School of Dentistry bridging fund (HX).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Wang, L., Xie, X., Weir, M.D. et al. Effect of bioactive dental adhesive on periodontal and endodontic pathogens. J Mater Sci: Mater Med 27, 168 (2016). https://doi.org/10.1007/s10856-016-5778-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5778-2