Abstract
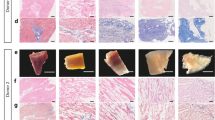
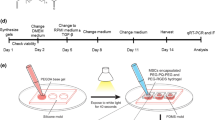
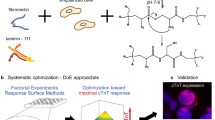
Freshly isolated human cardiac extracellular matrix sheets (cECM) have been shown to support stem cell proliferation and tissue-specific lineage commitment. We now developed a protocol for standardized production of durable, bio-functional hcECM microparticles and corresponding hydrogel, and tested its cytoprotective effects on contractile cells subjected to ischemia-like conditions. Human ventricular myocardium was decellularized by a 3-step protocol, including Tris/EDTA, SDS and serum incubation (cECM). Following snap-freezing and lyophilization, microparticles were created and characterized by laser diffraction, dynamic image analysis (DIA), and mass spectrometry. Moreover, cECM hydrogel was produced by pepsin digestion. Baseline cell-support characteristics were determined using murine HL-1 cardiomyocytes, and the cytoprotective effects of ECM products were tested under hypoxia and glucose/serum deprivation. In cECM, glycoproteins (thrombospondin 1, fibronectin, collagens and nidogen-1) and proteoglycans (dermatopontin, lumican and mimecan) were preserved, but residual intracellular and blood-borne proteins were also detected. The median particle feret diameter was 66 μm (15–157 μm) by laser diffraction, and 57 μm (20–182 μm) by DIA with crystal violet staining. HL-1 cells displayed enhanced metabolic activity (39 ± 12 %, P < 0.05) and proliferation (16 ± 3 %, P < 0.05) when grown on cECM microparticles in normoxia. During simulated ischemia, cECM microparticles exerted distinct cytoprotective effects (MTS conversion, 240 ± 32 %; BrdU uptake, 45 ± 14 %; LDH release, −72 ± 7 %; P < 0.01, each). When cECM microparticles were solubilized to form a hydrogel, the cytoprotective effect was initially abolished. However, modifying the preparation process (pepsin digestion at pH 2 and 25 °C, 1 mg/ml final cECM concentration) restored the cytoprotective cECM activity. Extracellular matrix from human myocardium can be processed to yield standardized durable microparticles that exert specific cytoprotective effects on cardiomyocyte-like cells. The use of processed cECM may help to optimize future clinical-grade myocardial tissue engineering approaches.






Similar content being viewed by others
References
Oberwallner B, Brodarac A, Anic P, Saric T, Wassilew K, Neef K, Choi YH, Stamm C. Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. Eur J Cardio-Thorac Surg. 2015;47:416–25 (discussion 425).
Oberwallner B, Brodarac A, Choi YH, Saric T, Anic P, Morawietz L, Stamm C. Preparation of cardiac extracellular matrix scaffolds by decellularization of human myocardium. J Biomed Mater Res Part A. 2014;102:3263–72.
Dai W, Gerczuk P, Zhang Y, Smith L, Kopyov O, Kay GL, Jyrala AJ, Kloner RA. Intramyocardial injection of heart tissue-derived extracellular matrix improves postinfarction cardiac function in rats. J Cardiovasc Pharmacol Ther. 2013;18:270–9.
Godier-Furnemont AF, Martens TP, Koeckert MS, Wan L, Parks J, Arai K, Zhang G, Hudson B, Homma S, Vunjak-Novakovic G. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci USA. 2011;108:7974–9.
Gui L, Chan SA, Breuer CK, Niklason LE. Novel utilization of serum in tissue decellularization. Tissue Eng Part C Methods. 2010;16:173–84.
Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A. 2010;16:2017–27.
Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16.
Zabel C, Klose J. High-resolution large-gel 2de. Methods Mol Biol. 2009;519:311–38.
Zabel C, Klose J. Protein extraction for 2de. Methods Mol Biol. 2009;519:171–96.
Hartl D, Rohe M, Mao L, Staufenbiel M, Zabel C, Klose J. Impairment of adolescent hippocampal plasticity in a mouse model for alzheimer’s disease precedes disease phenotype. PLoS One. 2008;3:e2759.
Mie G. Beiträge zur optik trüber medien, speziell kolloidaler metallösungen. Ann Phys. 1908;4:377–445.
Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ Jr. Hl-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–84.
Bader AM, Brodarac A, Klose K, Bieback K, Choi YH, Kurtz A, Stamm C. Mechanisms of paracrine cardioprotection by cord blood mesenchymal stromal cells. Eur J Cardio-Thorac Surg. 2014;45:983–92.
Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93.
Eitan Y, Sarig U, Dahan N, Machluf M. Acellular cardiac extracellular matrix as a scaffold for tissue engineering: in-vitro cell support, remodeling and biocompatibility. Tissue Eng Part C Methods. 2009;16:671–83.
Hata H, Bar A, Dorfman S, Vukadinovic Z, Sawa Y, Haverich A, Hilfiker A. Engineering a novel three-dimensional contractile myocardial patch with cell sheets and decellularised matrix. Eur J Cardiothorac Surg. 2010;38:450–5.
Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21.
Bayrak A, Tyralla M, Ladhoff J, Schleicher M, Stock UA, Volk HD, Seifert M. Human immune responses to porcine xenogeneic matrices and their extracellular matrix constituents in vitro. Biomaterials. 2010;31:3793–803.
Brodarac A, Saric T, Oberwallner B, Mahmoodzadeh S, Neef K, Albrecht J, Burkert K, Oliverio M, Nguemo F, Choi YH, Neiss WF, Morano I, Hescheler J, Stamm C. Susceptibility of murine induced pluripotent stem cell-derived cardiomyocytes to hypoxia and nutrient deprivation. Stem Cell Res Ther. 2015;6:83.
Hughes BG, Schulz R. Targeting mmp-2 to treat ischemic heart injury. Basic Res Cardiol. 2014;109:424.
Takawale A, Fan D, Basu R, Shen M, Parajuli N, Wang W, Wang X, Oudit GY, Kassiri Z. Myocardial recovery from ischemia-reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circ Heart Fail. 2014;7:652–62.
Hsu PL, Su BC, Kuok QY, Mo FE. Extracellular matrix protein ccn1 regulates cardiomyocyte apoptosis in mice with stress-induced cardiac injury. Cardiovasc Res. 2013;98:64–72.
Law CH, Li JM, Chou HC, Chen YH, Chan HL. Hyaluronic acid-dependent protection in h9c2 cardiomyocytes: a cell model of heart ischemia-reperfusion injury and treatment. Toxicology. 2013;303:54–71.
Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85:413–23.
Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5:173ra125.
Acknowledgments
We thank Dr. Katharina Wassilew for her help with preliminary experiments and Mrs. Anne Gale for copy-editing this manuscript. The work was supported with grants from the Federal Ministry of Education and Research and the federal states of Berlin and Brandenburg (FKZ 1315848A, FKZ 13GW0099).
Author information
Authors and Affiliations
Corresponding author
Additional information
Benjamin Kappler and Petra Anic have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kappler, B., Anic, P., Becker, M. et al. The cytoprotective capacity of processed human cardiac extracellular matrix. J Mater Sci: Mater Med 27, 120 (2016). https://doi.org/10.1007/s10856-016-5730-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5730-5