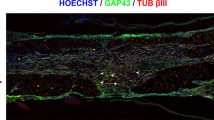
Abstract
Currently, there is no universally accepted treatment for traumatic spinal cord injury (TSCI), a pathology that can cause paraplegia or quadriplegia. Due to the complexity of TSCI, more than one therapeutic strategy may be necessary to regain lost functions. Therefore, the present study proposes the use of implants of mesoparticles (MPs) of polypyrrole/iodine (PPy/I) synthesized by plasma for neuroprotection promotion and functional recovery in combination with treadmill training (TT) for neuroplasticity promotion and maintenance of muscle tone. PPy/I films were synthesized by plasma and pulverized to obtain MPs. Rats with a TSCI produced by the NYU impactor were divided into four groups: Vehicle (saline solution); MPs (PPy/I implant); Vehicle-TT (saline solution + TT); and MPs-TT (PPy/I implant + TT). The vehicle or MPs (30 μL) were injected into the lesion site 48 h after a TSCI. Four days later, TT was carried out 5 days a week for 2 months. Functional recovery was evaluated weekly using the BBB motor scale for 9 weeks and tissue protection using histological and morphometric analysis thereafter. Although the MPs of PPy/I increased nerve tissue preservation (P = 0.03) and promoted functional recovery (P = 0.015), combination with TT did not produce better neuroprotection, but significantly improved functional results (P = 0.000) when comparing with the vehicle group. So, use these therapeutic strategies by separately could stimulate specific mechanisms of neuroprotection and neuroregeneration, but when using together they could mainly potentiate different mechanisms of neuronal plasticity in the preserved spinal cord tissue after a TSCI and produce a significant functional recovery.
Graphical Abstract
The implant of mesoparticles of polypyrrole/iodine into the injured spinal cord displayed good integration into the nervous tissue without a response of rejection, as well as an increased in the amount of preserved tissue and a better functional recovery than the group without transplant after a traumatic spinal cord injury by contusion in rats. The relevance of the present results is that polypyrrole/iodine implants were synthesized by plasma instead by conventional chemical or electrochemical methods. Synthesis by plasma modifies physicochemical properties of polypyrrole/iodine implants, which can be responsible of the histological response and functional results. Furthermore, no additional molecules or trophic factors or cells were added to the implant for obtain such results. Even more, when the implant was used together with physical rehabilitation, better functional recovery was obtained than that observed when these strategies were used by separately.






Similar content being viewed by others
References
Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26(24 Suppl.):S2–12.
Raineteau O. Plastic responses to spinal cord injury. Behav Brain Res. 2008;192:114–23.
Cafferty WB, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 2004;24(18):4432–43.
Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23:453–67.
Levi AD, Dancausse H, Li X, Duncan S, Horkey L, Oliviera M. Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg. 2002;96(2 Suppl):197–205.
Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–44.
Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–25.
Straley KS, Foo CW, Heilshorn SC. Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma. 2010;27(1):1–19.
Wang M, Zhaid P, Chen X, Schreyer DJ, Sun X, Cui F. Bioengineered scaffolds for spinal cord repair. Tissue Eng Part B Rev. 2011;17:177–94.
Josten EA. Biodegradable biomatrices and bridging the injured spinal cord: the corticospinal tract as a proof of principle. Cell Tissue Res. 2012;349:375–95.
Ahn HS, Hwang JY, Kim MS, Lee JY, Kim JW, Kim HS, Shin US, et al. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015;13:324–34.
Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai H, Ohta M, Wu S, Suzuki K, et al. Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng. 2004;10:493–504.
Stokols S, Tuszynski MH. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27:443–51.
Chen J, Zhang Z, Liu J, Zhou R, Zheng X, Chen T, Wang L, et al. Acellular spinal cord scaffold seeded with bone marrow stromal cells protects tissue and promotes functional recovery in spinal cord-injured rats. J Neurosci Res. 2014;92(3):307–17.
Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J Neurosurg Spine. 2004;1:322–9.
Hejcl A, Urdzikova L, Sedy J, Lesny P, Pradny M, Michalek J, Burian M, et al. Acute and delayed implantation of positively charged 2-hydroxyethyl methacrylate scaffolds in spinal cord injury in the rat. J Neurosurg Spine. 2008;8:67–73.
Woerly S, Doan VD, Sosa N, de Vellis J, Espinosa-Jeffrey A. Prevention of gliotic scar formation by NeuroGel allows partial endogenous repair of transected cat spinal cord. J Neurosci Res. 2004;75:262–72.
Olayo R, Rios C, Salgado-Ceballos H, Cruz GJ, Morales J, Olayo MG, Alcaraz-Zubeldia M, et al. Tissue spinal cord response in rats after implants of polypyrrole and polyethylene glycol obtained by plasma. J Mater Sci Mater Med. 2008;19:817–26.
Cruz GJ, Mondragón-Lozano R, Diaz-Ruiz A, Manjarrez J, Olayo R, Salgado-Ceballos H, Olayo MG, et al. Plasma polypyrrole implants recover motor function in rats after spinal cord transection. J Mater Sci Mater Med. 2012;23:2583–92.
Zamani F, Amani-Tehran M, Latifi M, Shokrgozar MA, Zaminy A. Promotion of spinal cord axon regeneration by 3D nanofibrous core-sheath scaffolds. J Biomed Mater Res A. 2014;102(2):506–13.
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, et al. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J Tissue Eng Regen Med. 2011;5(4):e17–35.
Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81:135–49.
Cruz GJ, Olayo MG, López OG, Gomez LM, Morales J, Olayo R. Nanospherical particles of polypyrrole synthesized and doped by plasma. Polymer. 2010;51:4314–8.
Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–95.
Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72.
Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. J Rehabil Res Dev. 2008;45:229–40.
Nessler JA, De Leon RD, Sharp K, Kwak E, Minakata K, Reinkensmeyer DJ. Robotic gait analysis of bipedal treadmill stepping by spinal contused rats: characterization of intrinsic recovery and comparison with BBB. J Neurotrauma. 2006;23:882–96.
Knikou M. Plasticity of corticospinal neural control after locomotor training in human spinal cord injury. Neural Plast. 2012. doi:10.1155/2012/254948.
Martinez M, Delivet-Mongrain H, Rossignol S. Treadmill training promotes spinal changes leading to locomotor recovery after partial spinal cord injury in cats. J Neurophysiol. 2013;109:2909–22.
Ley General de Salud de la República Mexicana. 6th ed. México D.F.: Porrúa; 1995.
Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21.
Morales J, Olayo MG, Cruz GJ, Olayo R. Synthesis by plasma and characterization of bi-layer aniline-pyrrole thin films doped with iodine. J Polym Sci Part B. 2002;40:1850–6.
Krishna V, Konakondla S, Nicholas J, Varma A, Kindy M, Wen X. Biomaterial-based interventions for neuronal regeneration and functional recovery in rodent model of spinal cord injury: a systematic review. J Spinal Cord Med. 2013;36(3):174–90.
Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006;201:359–67.
King VR, Alovskaya A, Wei DY, Brown RA, Priestley JV. The use of injectable forms of fibrin and fibronectin to support axonal ingrowth after spinal cord injury. Biomaterials. 2010;31:4447–56.
Cigognini D, Satta A, Colleoni B, Silva D, Donegà M, Antonini S, Gelain F. Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self-assembling scaffold. PLoS ONE. 2011;6:e19782.
Tsai EC, Dalton PD, Shoichet MS, Tator CH. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials. 2006;27:519–33.
Fine EG, Valentini RF, Bellamkonda R, Aebischer P. Improved nerve regeneration through piezoelectric vinylidene fluoride-trivfluoroethylene copolymer guidance channels. Biomaterials. 1991;12:775–80.
Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22:1055–64.
Zhang Z, Rouabhia M, Wang Z, Roberge C, Shi G, Roche P, Li J, et al. Electrically conductive biodegradable polymer composite for nerve regeneration: electricity-stimulated neurite outgrowth and axon regeneration. Artif Organs. 2007;31:13–22.
HajjHassan M, Chodavarapu V, Musallam S. Neuro MEMS: neural probe microtechnologies. Sensors. 2008;8:6704–26.
Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, Magnuson DSK. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma. 2009;26:1017–27.
Nothias JM, Mitsui T, Shumsky JS, Fischer I, Antonacci MD, Murray M. Combined effects of neurotrophin secreting transplants, exercise, and serotonergic drug challenge improve function in spinal rats. Neurorehabil Neural Repair. 2005;19:296–312.
Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34.
Engesser-Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW, Anderson AJ. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur J Neurosci. 2007;25:1931–9.
Grasso R, Ivanenko YP, Zago M, Molinari M, Scivoletto G, Castellano V, Macellari V, et al. Distributed plasticity of locomotor pattern generators in spinal cord injured patients. Brain. 2004;127:1019–34.
Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, et al. Training locomotor networks. Brain Res Rev. 2008;57:241–54.
Moshonkina T, Avelev V, Gerasimenko Y, Mathur R, Bijlani RL. Treadmill training accelerates restoration of locomotion after complete spinal cord transection in the rat. Indian J Physiol Pharmacol. 2002;46:499–503.
Oh MJ, Seo TB, Kwon KB, Yoon SJ, Elzi DJ, Kim BG, Namgung U. Axonal outgrowth and Erk1/2 activation by training after spinal cord injury in rats. J Neurotrauma. 2009;26:2071–82.
Foret A, Quertainmont R, Botman O, Bouhy D, Amabili P, Brook G, Schoenen J, et al. Stem cells in the adult rat spinal cord: plasticity after injury and treadmill training exercise. J Neurochem. 2010;112:762–72.
Sun T, Ye C, Wu J, Zhang Z, Cai Y, Yue F. Treadmill step training promotes spinal cord neural plasticity after incomplete spinal cord injury. Neural Regen Res. 2013;8:2540–7.
Acknowledgments
The work was supported by Instituto Mexicanos del Seguro Social (IMSS), Grant No. FIS/IMSS/PROT/G11/943 and by Consejo Nacional de Ciencia y Tecnología (CONACyT), Grant No. 47467. Laura Alvarez received a scholarship from CONACyT (No. 172211). The authors thank María del Carmen Baltazar for her invaluable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest relevant to this article is reported.
Rights and permissions
About this article
Cite this article
Alvarez-Mejia, L., Morales, J., Cruz, G.J. et al. Functional recovery in spinal cord injured rats using polypyrrole/iodine implants and treadmill training. J Mater Sci: Mater Med 26, 209 (2015). https://doi.org/10.1007/s10856-015-5541-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5541-0