Abstract
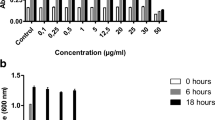
Orthopedic implant failure as a result of bacterial infection affects approximately 0.5–5 % of patients. These infections are often caused by Staphylococcus aureus which is capable of attaching and subsequently forming a biofilm on the implant surface, making it difficult to eradicate with systemic antibiotics. Further, with the emergence of antibiotic-resistant bacteria, alternative treatments are necessary. Silver nanoparticles have received much attention for their broad spectrum antibacterial activity which has been reported to be both size and shape dependent. The purpose of this study was therefore to evaluate the effect of three different geometries on their effect on microbial susceptibility as well as evaluate their effect on bone cell viability. Silver nanoparticles of spherical, triangular and cuboid shapes were synthesized by chemical reduction methods. The susceptibility of S. aureus and methicillin-resistant S. aureus was evaluated a 24 h period and determined using a colorimetric assay. Further, the viability of human fetal osteoblast (hFOB) cells in the presence of the silver nanoparticles was evaluated over a period of 7 days by AlmarBlue fluorescence assay. hFOB morphology was also evaluated by light microscopy imaging. Results indicated that silver nanoparticle geometry did not have an effect on microbiota susceptibility or hFOB viability. However, high concentrations of silver nanoparticles (0.5 nM) conferred significant susceptibility towards the bacteria and significantly reduced hFOB viability. It was also found that the hFOBs exhibited an increasingly reduced viability to lower silver nanoparticle concentrations with an increase in exposure time.





Similar content being viewed by others
References
Campoccia D, Montanaro L, Arciola CR. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials. 2013;34:8018–29.
Evan M, Hetrick MHS. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–9.
Romeo T, editor. Bacterial biofilms. Current topics in microbiology and immunology, vol. 322. Berlin: Springer; 2008.
Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–56.
Flemming H-C. Biofilm highlights. Springer Series on Biofilms. New York: Springer; 2011.
Kawata K, Osawa M, Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ Sci Technol. 2009;43(15):6046–51.
Zimmerli W. Prosthetic-join-associated infections. Best Pract Res Clin Rheumatol. 2006;20(6):1045–63.
Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter. 2012;2(4):176–94.
Leid JG, Leid J, Shirtliff M, editors. The role of biofilm in device-related infections. Springer Series on Biofilms. Los Angeles: Springer; 2009.
Vasilev K, Cook J, Griesser HJ. Antibacterial surfaces for biomedical devices. Exp Rev Med Devices. 2009;6(5):553–67.
Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol. 2011;9(1):30.
Tran QH, Le AT. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv Nat Sci Nanosci Nanotechnol. 2013;4:033001.
Dal Lago V, de Oliveira LF, de Almeida Gonçalves K, Kobarg J, Cardoso MB. Size-selective silver nanoparticles: future of biomedical devices with enhanced bactericidal properties. J Mater Chem. 2011;21(33):12267–73.
Marambio-Jones C, Hoek EM. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res. 2010;12:1531–51.
Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–20.
Metraux GS, Mirkin CA. Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv Mater. 2005;17(4):412–5.
Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298:2176–9.
Yguerabide J, Yguerabide EE. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. Anal Biochem. 1998;262:137–56.
Chen Z, Gao L. A facile and novel way for the synthesis of nearly monodisperse silver nanoparticles. Mater Res Bull. 2007;42:1657–61.
Mansouri SS, Ghader S. Experimental study of effect of different parameters on size and shape of triangular silver nanoparticles prepared by a simple and rapid method in aqueous solution. Arab J Chem. 2009;2:47–53.
Chen S, Carroll DL. Silver nanoplates: size control in two dimensions and formulation mechanism. J Phys Chem B. 2004;108:5500–6.
Skrabalak SE, Au L, Li X, Xia Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat Protoc. 2007;2(9):2182–90.
Mdluli PS, Sosibo NM, Mashazi PN, Nyokong T, Tshikhudo RT, Skepu A, Van Der Lingen E. Selective adsorption of PVP on the surface of silver nanoparticles: a molecular dynamics study. J Mol Struct. 2011;1004:131–7.
Kvitek L, Panáček A, Soukupova J, Kolar M, Vecerova R, Prucek R, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C. 2008;112:5825–34.
Pauksch L, Hartmann S, Rohnke M, Szalay G, Alt V, Schnettler R, Lips KS. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014;10:439–49.
Tautzenberger A, Kovtun A, Ignatius A. Nanoparticles and their potential application in bone. Int J Nanomed. 2012;7:4545–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Actis, L., Srinivasan, A., Lopez-Ribot, J.L. et al. Effect of silver nanoparticle geometry on methicillin susceptible and resistant Staphylococcus aureus, and osteoblast viability. J Mater Sci: Mater Med 26, 215 (2015). https://doi.org/10.1007/s10856-015-5538-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5538-8