Abstract
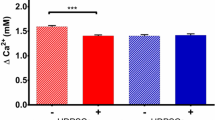
Calcium (Ca) is the main element of most pulp capping materials and plays an essential role in mineralization. Different pulp capping materials can release various concentrations of Ca ions leading to different clinical outcomes. The purpose of this study was to investigate the effects of various concentrations of Ca ions on the growth and osteogenic differentiation of human dental pulp cells (hDPCs). Different concentrations of Ca ions were added to growth culture medium and osteogenic inductive culture medium. A Cell Counting Kit-8 was used to determine the proliferation of hDPCs in growth culture medium. Osteogenic differentiation and mineralization were measured by alkaline phosphatase (ALP) assay, Alizarin red S/von kossa staining, Ca content quantitative assay. The selected osteogenic differentiation markers were investigated by quantitative real-time polymerase chain reaction (qRT-PCR). Within the range of 1.8–16.2 mM, increased concentrations of Ca ions had no effect on cell proliferation, but led to changes in osteogenic differentiation. It was noted that enhanced mineralized matrix nodule formation was found in higher Ca ions concentrations; however, ALP activity and gene expression were reduced. qRT-PCR results showed a trend towards down-regulated mRNA expression of type I collagen and Runx2 at elevated concentrations of Ca ions, whereas osteopontin and osteocalcin mRNA expression were significantly up-regulated. Ca ions content in the culture media can significantly influence the osteogenic properties of hDPCs, indicating the importance of optimizing Ca ions release from dental pulp capping materials in order to achieve desirable clinical outcomes.




Similar content being viewed by others
References
Hilton TJ. Keys to clinical success with pulp capping: a review of the literature. Oper Dent. 2009;34:615–25. Review.
Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92.
Scarano A, Manzon L, Di Giorgio R, Orsini G, Tripodi D, Piattelli A. Direct capping with four different materials in humans: histological analysis of odontoblast activity. J Endod. 2003;29:729–34.
Hörsted-Bindslev P, Vilkinis V, Sidlauskas A. Direct capping of human pulps with a dentin bonding system or with calcium hydroxide cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:591–600.
Duarte MA, Martins CS, de Oliveira Cardoso Demarchi AC, de Godoy LF, Kuga MC, Yamashita JC. Calcium and hydroxide release from different pulp-capping materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:66–9.
Estrela C, Sydney GB, Bammann LL, Felippe Júnior O. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1995;6:85–90.
Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, Ito K. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 2006;39:415–22.
Kiba W, Imazato S, Takahashi Y, Yoshioka S, Ebisu S, Nakano T. Efficacy of polyphasic calcium phosphates as a direct pulp capping material. J Dent. 2010;38:828–37.
Shen Q, Sun J, Wu J, Liu C, Chen F. An in vitro investigation of the mechanical-chemical and biological properties of calcium phosphate/calcium silicate/bismutite cement for dental pulp capping. J Biomed Mater Res B Appl Biomater. 2010;94:141–8.
Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J. 2003;36:225–31.
Mente J, Geletneky B, Ohle M, Koch MJ, Friedrich Ding PG, et al. Mineral trioxide aggregate or calcium hydroxide direct pulp capping: an analysis of the clinical treatment outcome. J Endod. 2010;36:806–13.
Rashid F, Shiba H, Mizuno N, Mouri Y, Fujita T, Shinohara H, Ogawa T, Kawaguchi H, Kurihara H. The effect of extracellular calcium ion on gene expression of bone-related proteins in human pulp cells. J Endod. 2003;29:104–7.
Wei X, Ling J, Wu L, Liu L, Xiao Y. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33:703–8.
Tada H, Nemoto E, Kanaya S, Hamaji N, Sato H, Shimauchi H. Elevated extracellular calcium increases expression of bone morphogenetic protein-2 gene via a calcium channel and ERK pathway in human dental pulp cells. Biochem Biophys Res Commun. 2010;394:1093–7.
Ahlstrom M, Pekkinen M, Riehle U, Lamberg-Allardt C. Extracellular calcium regulates parathyroid hormone-related peptide expression in osteoblasts and osteoblast progenitor cells. Bone. 2008;42:483–90.
Sharan K, Siddiqui JA, Swarnkar G, Chattopadhyay N. Role of calcium-sensing receptor in bone biology. Indian J Med Res. 2008;127:274–86.
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–9.
Finan JD, Guilak F. The effects of osmotic stress on the structure and function of the cell nucleus. J Cell Biochem. 2010;109:460–7.
Dmitrieva NI, Michea LF, Rocha GM, Burg MB. Cell cycle delay and apoptosis in response to osmotic stress. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:411–20.
Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, Toyama Y, Taguchi T, Tanaka J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials. 2005;26:4847–55.
Nakamura S, Matsumoto T, Sasaki JI, Egusa H, Lee KY, Nakano T, Sohmura T, Nakahira A. Effect of calcium ion concentrations on osteogenic differentiation and hematopietic stem cell niche-related protein expression in osteoblasts. Tissue Eng Part A. 2010;16:2467–73.
Eklou-Kalonji E, Denis I, Lieberherr M, Pointillart A. Effects of extracellular calcium on the proliferation and differentiation of porcine osteoblasts in vitro. Cell Tissue Res. 1998;292:163–71.
Sugimoto T, Kanatani M, Kano J, Kaji H, Tsukamoto T, Yamaguchi T, Fukase M, Chihara K. Effects of high calcium concentration on the functions and interactions of osteoblastic cells and monocytes and on the formation of osteoclast-like cells. J Bone Miner Res. 1993;8:1445–52.
Honda Y, Fitzsimmons RJ, Baylink DJ, Mohan S. Effects of extracellular calcium on insulin-like growth factor II in human bone cells. J Bone Miner Res. 1995;10:1660–5.
Ninomiya M, Ohishi M, Kido J, Ohsaki Y, Nagata T. Immunohistochemical localization of osteopontin in human pulp stones. J Endod. 2001;27:269–72.
Yokota M, Nagata T, Ishida H, Wakano Y. Clonal dental pulp cells (RDP4–1, RPC-C2A) synthesize and secrete osteopontin (SPP1, 2ar). Biochem Biophys Res Commun. 1992;189:892–8.
Mizuno M, Banzai Y. Calcium ion release from calcium hydroxide stimulated fibronectin gene expression in dental pulp cells and the differentiation of dental pulp cells to mineralized tissue forming cells by fibronectin. Int Endod J. 2008;41:933–8.
Pameijer CH, Stanley HR. The disastrous effects of the “total etch” technique in vital pulp capping in primates. Am J Dent. 1998;11:S45–54.
Shui C, Spelsberg TC, Riggs BL, Khosla S. Changes in Runx2/Cbfa1 expression and activity during osteoblastic differentiation of human bone marrow stromal cells. J Bone Miner Res. 2003;18:213–21.
Garcia JM, Martins MD, Jaeger RG, Marques MM. Immunolocalization of bone extracellular matrix proteins (type I collagen, osteonectin and bone sialoprotein) in human dental pulp and cultured pulp cells. Int Endod J. 2003;36:404–10.
Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int. 2000;66:129–38.
Disclosure
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shaofeng An and Yan Gao are contributed equally to this study.
Rights and permissions
About this article
Cite this article
An, S., Gao, Y., Ling, J. et al. Calcium ions promote osteogenic differentiation and mineralization of human dental pulp cells: implications for pulp capping materials. J Mater Sci: Mater Med 23, 789–795 (2012). https://doi.org/10.1007/s10856-011-4531-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4531-0