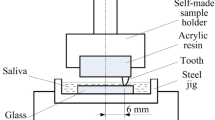
Abstract
The design and development of glass ceramic materials provide us the unique opportunity to study the microstructure development with changes in either base glass composition or heat treatment conditions as well as to understand processing-microstructure-property (mechanical/biological) relationship. In the present work, it is demonstrated how various crystal morphology can develop when F− content in base glass (K2O–B2O3–Al2O3–SiO2–MgO–F) is varied in the range of 1.08–3.85% and when all are heat treated at varying temperatures of 1000–1120°C. For some selected heat treatment temperature, the heat treatment time is also varied over 4–24 h. It was established that with increase in fluoride content in the glass composition, the crystal volume fraction of the glass-ceramic decreases. Using 1.08% fluoride, more than 80% crystal volume fraction could be achieved in the K2O–B2O3–Al2O3–SiO2–MgO–F system. It was observed that with lower fluoride content glass-ceramic, if heated at 1040°C for 12 h, an oriented microstructure with ‘envelop like’ crystals can develop. For glass ceramics with higher fluorine content (2.83% or 3.85%), hexagonal-shaped crystals are formed. Importantly, high hardness of around 8 GPa has been measured in glass ceramics with maximum amount of crystals. The three-point flexural strength and elastic modulus of the glass-ceramic (heat treated at 1040°C for 24 h) was 80 MPa and 69 GPa of the sample containing 3.85% fluorine, whereas, similar properties obtained for the sample containing 1.08% F− was 94 MPa and 57 GPa, respectively. Further, in vitro dissolution study of the all three glass-ceramic composition in artificial saliva (AS) revealed that leached fluoride ion concentration was 0.44 ppm, when the samples were immersed in AS for 8 weeks. This was much lower than the WHO recommended safety limits of 1.5 ppm. Among all the investigated glass-ceramic samples, the glass ceramic with 3.85% F− content in base glass (heat treated at 1040°C for 12 h), exhibits the adherence of Ca–P layer, which consists of spherical particles of 2–3 μm. Other ions, such as Mg+2 and K+1 ion concentrations in the solution were found to be 8 and 315 ppm after 8 weeks of leaching, respectively. The leaching of all metal ions is recorded to decrease with time, probably due to time-dependent kinetic modification of sample surface. Summarizing, the present study illustrates that it is possible to obtain a good combination of crystallization, mechanical and in vitro dissolution properties with the careful selection of base glass composition and heat treatment conditions.










Similar content being viewed by others
References
G.H. Beall, in Advances in Nucleation and Crystallization in Glass, ed. by L.L. Hench, S.W. Freiman (American Ceramic Society, Columbus, OH, 1971), Special publication no. 5, pp. 251–261
A.R. Molla, Influence of ceramising treatment on the crystal shape, in vitro and tribological properties of machinable glass ceramics, M.Tech thesis, IIT Kanpur, India, July, 2007
T. Uno, T. Kasuga, S. Nakayama, A.J. Ikushima, Microstructure of mica-based nanocomposite glass-ceramics. J. Am. Ceram. Soc. 76(2), 539–541 (1993). Feb
D.S. Baik, K.S. No, J.S. Chun, Mechanical properties of mica glass-ceramics. J. Am. Ceram. Soc. 78(5), 1217–1222 (1995). doi:10.1111/j.1151-2916.1995.tb08472.x
S. Roy, B. Basu, Hardness properties and microscopic investigation of crack-crystal interaction in SiO2–MgO–Al2O3–K2O–B2O–F glass ceramic system. Mat. Sci. Eng. C (provisionally accepted, 2008)
S. Roy, B. Basu, On the development of two characteristically different crystal morphology in SiO2–MgO–Al2O3–K2O–B2O–F glass-ceramic system. J. Mater. Sci. Mater. Med. http://dx.doi.org/10.1007/s10856-008-3536-9, in press
S. Roy, B. Basu, In vitro dissolution behaviour of SiO2–MgO–Al2O3–K2O–B2O–F glass-ceramic system. J. Mater. Sci.: Mater. Med. 19, 3123–3133 (2008). doi:10.1007/s10856-008-3440-3
S. Roy, B. Basu, Mechanical and tribological characterization of human tooth. Mater. Charact. 59, 747–756 (2008). doi:10.1016/j.matchar.2007.06.008
L.L. Hench, Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 74(7), 1487–1510 (1991). doi:10.1111/j.1151-2916.1991.tb07132.x
W. Holland, W. Vogel, An Introduction to Bioceramics (World Scientific Publishing Company Pvt Ltd, Singapore, 1993), pp. 125–137
W. Holand, G. Beall, Glass Ceramic Technology (The American Ceramic Society, Westerville, 2002)
T. Kokubo, S. Ito, M. Shigematsu, S. Sakka, T. Yamamuro, Mechanical properties of a new type of apatite-containing glass-ceramic for prosthetic application. J. Mater. Sci. 20, 2001–2004 (1985). doi:10.1007/BF01112282
T. Kokubo, S. Ito, S. Sakka, T. Yamamuro, Formation of a high-strength bioactive glass-ceramic in the system MgO–CaO–SiO2–P2O5. J. Mater. Sci. 21, 536–540 (1986). doi:10.1007/BF01145520
M. Akao, H. Aoki, K. Kato, Mechanical properties of sintered. hydroxyapatite for prosthetic application. J. Mater. Sci. 16, 809–812 (1981). doi:10.1007/BF02402799
G. Dewith, H.J.A. Vandijk, N. Hattu, K. Prijs, Preparation, microstructure and mechanical-properties of dense polycrystalline hydroxy apatite. J. Mater. Sci. 16, 1592–1598 (1981)
D.-M. Liu, Bioactive glass-ceramic: formation, characterization and bioactivity. Mater. Chem. Phys. 36, 294–303 (1994). doi:10.1016/0254-0584(94)90045-0
P. Vincenzini (ed.), Ceramics in Clinical Applications (Elsevier, Amsterdam, 1987)
T. Kitsugi, T. Yamamuro, T. Nakamura, T. Kokubo, Bone bonding behavior of MgO–CaO–SiO2–P2O5–CaF2 glass (mother glass of AW-glass-ceramics). J. Biomed. Mater. Res. 23, 631–648 (1989). doi:10.1002/jbm.820230607
T. Nakamuro, T. Yamamuro, S. Higashi, T. Kokubo, S. Ito, A new glass-ceramic for bone replacement: evaluation of its bonding to bone tissue. J. Biomed. Mater. Res. 19, 685 (1985)
J.B. Quinn, V. Sundar, I.K. Lloyd, Influence of microstructure and chemistry on the fracture toughness of dental ceramics. Dent. Mater. 19, 603–611 (2003). doi:10.1016/S0109-5641(03)00002-2
D.G. Grossman, Processing of dental ceramic by casting method. Ceram. Eng. Sci. Proc. 6, 19–40 (1985). doi:10.1002/9780470320259.ch3
X. Chen, L.L. Hench, D. Greenspan, Investigation on phase separation, nucleation and crystallization in bioactive glass-ceramics containing fluorophlogopite and fluorapatite. Ceram. Int. 24, 401–410 (1998). doi:10.1016/S0272-8842(97)00028-X
L. Radonjić, L. Nikolić, The effect of fluorine source and concentration on the crystallization of machinable glass-ceramics. J. Eur. Ceram. Soc. 7(1), 11–16 (1991)
K. Cheng, J. Wan, K. Liang, Crystallization of R2O–MgO–Al2O3–B2O3–SiO2–F (R = K+, Na+) glasses with different fluorine source. Mater. Lett. 47, 1–6 (2001). doi:10.1016/S0167-577X(00)00201-9
K.J. Anusavice, N.Z. Zhang, Chemical durability of Dicor and Lithia based glass ceramics. Dent. Mater. 13, 13–19 (1997)
L.A. Flanders, J.B. Quinn, C. Otto, C. Otto, Wilson Jr., I.K. Lloyd, Scratch hardness and chipping of dental ceramics under different environments. Dent. Mater. 19, 716–724 (2003). doi:10.1016/S0109-5641(03)00018-6
H. Li, Z.R. Zhou, Wear behaviour of human teeth in dry and artificial saliva conditions. Wear 249, 980–984 (2002). doi:10.1016/S0043-1648(01)00835-3
V. Saraswati, S. Raoot, Machinable mica-based glass-ceramic. J. Mater. Sci. 27, 429–432 (1992). doi:10.1007/BF00543933
T. Furukawa, W.B. White, Raman spectroscopy of heat-treated B2O3–SiO2 Glasses. J. Am. Ceram. Soc. 64, 443 (1981). doi:10.1111/j.1151-2916.1981.tb09893.x
M.I. Alemany, P. Velasquez, M.A. de la Casa-Lillo, P.N. De Aza, Effect of materials’ processing methods on the ‘in vitro’ bioactivity of wollastonite glass-ceramic materials. J. Non-Cryst. Solid 351, 1716–1726 (2005). doi:10.1016/j.jnoncrysol.2005.04.062
Chyung CK, Beall GH, Grossman DG, in 10th International congress on glass, vol 14 (reprinted by Ceramic Society of Japan, Kyoto, Japan, 1974), p. 33
M.S. Bapna, H.J. Mueller, Study of devitrification of Dicor® glass. Biomaterials 17, 2045–2052 (1996). doi:10.1016/0142-9612(96)00024-5
S. Habelitz, T. Höche, R. Hergt, G. Carl, C. Rüssel, Microstructural design through epitaxial growth in extruded mica glass-ceramics. Acta Mater. 47(9), 2831–2840 (1999). doi:10.1016/S1359-6454(99)00135-4
T. Höche, S. Habelitz, I. Avramov, Crystal morphology engineering in SiO2–Al2O3–MgO–K2O–Na2O–F− mica glass-ceramics. Acta Mater. 47(3), 735–744 (1999). doi:10.1016/S1359-6454(98)00424-8
X.P. Ma, G.X. Li, L. Shen, Z.H. Jin, Ductile-mode material removal of a mica–glass-ceramic. J. Am. Ceram. Soc. 86(6), 1040–1042 (2003)
A.C.F. Cripps, B.R. And Lawn, Indentation stress-strain curves for “quasi-ductile” ceramics. Acta Mater. 44(2), 519–527 (1996). doi:10.1016/1359-6454(95)00204-9
H. Tsuchiya, Y. Hoshino, K. Tajima, N. Takagi, Leaching and cytotoxicity of formaldehyde and methyl methacrylate from acrylic resin denture base materials. J. Prosthet. Dent. 71(December), 619–623 (1994).
W. Vogel, W. Höland, K. Naumann, J. Gummel, Development of machineable bioactive glass ceramics for medical uses. J. Non-Crystal. Solid 80(1–3), 34–51 (1986)
A. Gebhardt, T. Höche, G. Carl, I.I. Khodos, TEM study on the origin of cabbage-shaped mica crystal aggregates in machinable glass-ceramics. Acta Mater. 47(17), 4427–4434 (1999). doi:10.1016/S1359-6454(99)00317-1
W.A. Johnson, R.F. Mehl, Reaction Kinetics in process of nucleation and growth. Trans. AIME 135, 416–442 (1939)
M. Avrami, Kinetics of phase change, J. Chem. Phys. 7, 1103–1112 (1939), 9, 177–184 (1941)
M. Goswami, A. Sarkar, T. Mirza, V.K. Shrikhande, K.R. Sangeeta, Gurumurthy, G.P. Kothiyal, Study of some thermal and mechanical properties of magnesium aluminium silicate glass ceramic. Ceram. Int. 28, 585 (2002). doi:10.1016/S0272-8842(02)00013-5
D.S. Baik, K.S. No, J.S. Chun, H.Y. Cho, Effect of the aspect ratio of mica crystals and crystallinity on the microhardness and machinability of mica glass-ceramics. J. Mater. Process. Technol. 67, 50–54 (1997). doi:10.1016/S0924-0136(96)02817-8
W.B. Apambire, D.R. Boyle, F.A. Miche, Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ. Geol. 33(1), 13–24 (1997). doi:10.1007/s002540050221
L.L. Hench, R.J. Splinter, W.C. Allen, T.K. Greenlee Jr, Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 2, 117–141 (1972)
B.D. Ratner, A.S. Hoffman, F.J. Schoen, J.E. Lemons, Biomaterials Science: An Introduction to Materials in Medicine (Academic Press, London, 2004)
P.S. Wuttiphan, B.R. Lawn, K. Chyung, Role of microstructure on contact damage and strength degradation of micaceous glass-ceramics. Dent. Mater. 14(1), 80 (1998). doi:10.1016/S0109-5641(98)00013-X
J. Henry, R.G. Hill, The influence of lithia content on the properties of fluorphlogopite glass-ceramics. II. Microstructure hardness and machinability. J. Non-Cryst. Solid 319, 13 (2003). doi:10.1016/S0022-3093(02)01959-2
T. Kasuga, M. Yoshida, T. Uno, Preparation of zirconia toughened bioactive glass-ceramics. J Mater Sci 23, 2255–2258 (1988). doi:10.1007/BF01115797
W. Cao, L.L. Hench, Bioact. Mater. Ceram. Int. 22, 493–507 (1996). doi:10.1016/0272-8842(95)00126-3
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molla, A.R., Basu, B. Microstructure, mechanical, and in vitro properties of mica glass-ceramics with varying fluorine content. J Mater Sci: Mater Med 20, 869–882 (2009). https://doi.org/10.1007/s10856-008-3643-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3643-7