Abstract
Scaffolds from poly(ethylene oxide) and poly(butylene terephthalate), PEOT/PBT, with a PEO molecular weight of 1,000 and a PEOT content of 70 weight% (1000PEOT70PBT30) were prepared by leaching salt particles (425–500 μm). Scaffolds of 73.5, 80.6 and 85.0% porosity were treated with a CO2 gas plasma and seeded with rat bone marrow stromal cells (BMSCs). After in vitro culture for 7 days (d) in an osteogenic medium the scaffolds were subcutaneously implanted for 4 weeks in nude mice. Poly(d, l-lactide) (PDLLA) and biphasic calcium phosphate (BCP) scaffolds were included as references. After 4 weeks (wks) all scaffolds showed ectopic formation of bone and bone marrow. For the scaffolds of different porosities, no significant differences were observed in the relative amounts of bone (7–9%) and bone marrow (6–11%) formed, even though micro computed tomography (μ-CT) data showed considerable differences in accessible pore volume and surface area. 1000PEOT70PBT30 scaffolds with a porosity of 85% could not maintain their original shape in vivo. Surprisingly, 1000PEOT70PBT30 scaffolds with a porosity of 73.5% showed cartilage formation. This cartilage formation is most likely due to poorly accessible pores in the scaffolds, as was observed in histological sections. μ-CT data showed a considerably smaller accessible pore volume (as a fraction of the total volume) than in 1000PEOT70PBT30 scaffolds of 80.6 and 85.0% porosity. BMSC seeded PDLLA (83.5% porosity) and BCP scaffolds (29% porosity) always showed considerably more bone and bone marrow formation (bone marrow formation is approximately 40%) and less fibrous tissue ingrowth than the 1000PEOT70PBT30 scaffolds. The scaffold material itself can be of great influence. In more hydrophobic and rigid scaffolds like the PDLLA or BCP scaffolds, the accessibility of the pore structure is more likely to be preserved under the prevailing physiological conditions than in the case of hydrophilic 1000PEOT70PBT30 scaffolds. Scaffolds prepared from other PEOT/PBT polymer compositions, might prove to be more suited.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Large bone defects do not heal spontaneously and require surgical intervention for restoration. The inherent drawback of the use of autologous trabecular grafts, however, is that the grafts have to be removed from another place in the human body, resulting in donor-site morbidity [1]. A possible alternative is the use of allogeneic bone. This, however, shows a lower osteogenic capacity, a higher resorption rate, a larger immunogenic response and less extensive revascularization of the graft. Furthermore there are concerns over the possibility of viral contamination of the graft material and possible transmission of live virus to the recipient. The rapidly developing field of tissue engineering offers advantageous approaches for defect repair.
As scaffold materials, porous polymers have attracted much attention [2]. Due to the vast variety of preparation techniques, many different polymeric scaffold architectures can be obtained. The mechanical and physical properties of poly(ethylene oxide)/poly(butylene terephthalate) (PEOT/PBT) segmented block copolymers can be tuned by varying the PBT (hard segment) content and PEO (soft segment) content and molecular weight [3, 4]. These properties make these copolymers interesting candidates for use as scaffold materials in (bone) tissue engineering. Besides this, several subcutaneous and intra-bone (tibia) implantations of dense and porous blocks and porous films in rats and goats showed bonding to bone, calcification and degradation for PEOT/PBT copolymers with high PEO content (1000PEOT60PBT40 and 1000PEOT70PBT30, prepared from polyethylene glycol of molecular weight 1000 g/mol with respectively 60 and 70 wt% PEOT hydrophilic soft segments and 40 and 30 wt% hydrophobic PBT hard segments) [5–9]. However, after implantation of porous blocks of 1000PEOT70PBT30 in goat [10] and human [11] ilia critical size defects, poor bone bonding, limited calcification and limited fragmentation were, observed. It is anticipated that seeding 1000PEOT70PBT30 scaffolds with BMSCs will yield structures with osteoinductive properties [12] that are better suited for bone tissue engineering than the scaffolds without BMSCs.
The in vitro culture of (rat) bone marrow stromal cells (BMSCs) in an osteogenic medium containing dexamethasone, β-glycerophosphate and l-ascorbic acid greatly increases the amount of cells with an osteoblastic phenotype [13–16]. In many systems, seeding of BMSCs (after expansion in culture) on a porous scaffold, followed by a period of in vitro cell culture in an osteogenic medium prior to implantation, resulted in enhanced ectopic bone formation compared to scaffolds that were seeded and implanted immediately [17, 18].
Besides the culturing conditions, it was shown for PLGA scaffolds that scaffold morphology (i.e., pore size and porosity) can also influence the in vivo results [18]. Until now, the porosity of 1000PEOT70PBT30 scaffold materials has not been optimized for bone tissue engineering. To study the effect of porosity and accessible pore volume on ectopic bone formation, CO2 plasma treated 1000PEOT70PBT30 scaffolds of 73.5, 80.6 and 85.0 % porosity were prepared by leaching salt particles of 425–500 μm and subcutaneously implanted in nude mice after seeding with rat BMSCs and in vitro culture for 7 d (days) in an osteogenic medium. Ectopic bone formation was evaluated and quantified by histomorphometry.
Materials and methods
Materials
The 1000PEOT70PBT30 copolymer was prepared as previously described [19], PDLLA, inherent viscosity 2.96 dl/g, was obtained from Purac (Gorinchem, The Netherlands), and purified by precipitation. Porous structures were prepared by compression molding of polymer/salt mixtures followed by salt leaching. The salt particle size used was 425–500 μm. Scaffolds of 4 mm × 4 mm × 4 mm were cut with a razor blade and treated with a CO2 plasma [20]. Porosities were determined by measurement of scaffold mass and dimensions, using a density of ρ = 1.188 g/cm3 for solid 1000PEOT70PBT30 and ρ = 1.26 g/cm3 for PDLLA. Bicalcium phosphate granules [17] (OsSaturaTM, BCP) were characterized by mercury intrusion porosimetry and provided by Isotis OrthoBiologics (Bilthoven, The Netherlands).
Cell culturing and implantation
Bone marrow stromal cells were isolated from 16 femora of 8 young male Wistar-rats (100–120 g). The osteogenic culture medium was minimal essential medium (α-MEM, Life Technologies, The Netherlands) containing [13]: 15% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 0.2 mM ascorbic acid 2-phosphate, 10 mM β-glycerophosphate, 10−8 M dexamethasone.
The scaffolds were put in culture medium overnight and seeded with 2 × 150 μl of cell suspension (approximately 2 × 105 cells per scaffold). Cell suspensions were injected into the scaffolds with a pipette tip. Scaffolds were incubated at 37°C for 3 hours (h), after which 2 ml of cell culture medium was added. Cells were cultured at 37°C, 5 % CO2 for 7 d, with periodic medium changes every other day. At day 7 the samples were either implanted or analyzed using SEM or a DNA assay.
Cell-seeded and cultured scaffolds were subcutaneously implanted in six immunodeficient mice (six sites per mouse). After 4 weeks (wks) the mice were sacrificed and the scaffolds were removed and fixed with glutaraldehyde (1.5% solution in cacodylic acid buffer). One mouse died prematurely and the implanted scaffolds were excluded from the experiment. For each of the 1000PEOT70PBT30 scaffolds 5 samples were analyzed, unless otherwise mentioned.
Analyses
Compression moduli of scaffolds (height 8 mm, diameter 17 mm) were determined at 10% strain in triplicate at room temperature using a Zwick Z020 tensile tester operating at 2 mm/min.
Dry, unseeded scaffolds were scanned using a Micro-CT from Scanco Medical (Bassersdorf, Switzerland). The porosity is calculated from the number of polymer voxels and the total number of voxels. To determine the pore size, the diameter of a largest modeled sphere fitting in the (cubic) pore is assigned to all voxels within that pore.
An algorithm mimicking mercury intrusion porosimetry was used to determine the accessible pore volume, and its surface area was calculated using a triangularization algorithm. In a following paper the micro-CT analysis will be dealt with in more detail.
Scaffolds containing rat BMSCs were fixed, dehydrated and dried, then coated with Au/Pd and observed with a Hitachi FE-SEM S-800 scanning electron microscope (SEM).
To determine the DNA (and cell) content, scaffolds were washed with phosphate buffered saline (PBS) and incubated at 56°C overnight in 0.5 ml of proteinase K lysis-medium (Sigma, The Netherlands) to lyse all cells. Then 250 μl of these suspensions were mixed with 250 μl RNase-solution. (prepared from 30 μl RNase (Sigma, The Netherlands, 1.35 Kunitz units/μl) and 50 μl heparin (Leopharma, The Netherlands, 5,000 IE/ml) in 12.5 ml PBS, and incubated at 37°C for 60 min to remove single stranded RNA and DNA. Various dilutions were prepared with PBS and mixed with CyQUANT® dye. After 15 min, the fluorescence of the solutions in 96 well plates was measured. The intensities were correlated to the amount of DNA using a calibration curve of DNA (Sigma) dilutions of known concentration. Data shown are the result of triplicate measurements ± SD).
Scaffolds were dehydrated and subsequently embedded in poly(methyl methacrylate) (PMMA), then sections were prepared and stained using 1% methylene blue and 0.3% basic fuchsine solutions. Samples were evaluated histologically and histomorphometrically by light microscopy. Sections from the middle part of the scaffolds were analyzed at high magnifications and the relative amounts of the different tissues formed ectopically were calculated from the number of pixels using Scion Image software. Data shown are the average of 5 scaffolds ± SD, unless otherwise mentioned.
Results and discussion
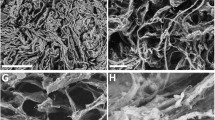
Six different scaffolds, seeded with rat BMSCs and cultured for 7 d in an osteogenic medium, were subcutaneously implanted in immunodeficient nude mice and evaluated for tissue formation. The used scaffolds are depicted in Fig. 1 and some of their main characteristics are summarized in Table 1.
3D generated computer images constructed from μ-CT scans of dry scaffolds, prior to gas plasma treatment and cell seeding. The images corresponding to scaffolds A, B, C, D, E and F (identical to A) as listed in Table 1 are shown
Three 1000PEOT70PBT30 scaffolds differing in porosity (scaffolds A, B and C) were treated using a CO2 plasma. Untreated 1000PEOT70PBT30 (scaffold F) seeded with rat BMSCs and cultured for 7 d in an osteogenic medium, were used as a negative control for in vivo bone formation. For comparison scaffolds were also prepared from PDLLA (scaffold D), a well-known biomaterial. Cell-seeded and cultured biphasic calcium phosphate (OsSaturaTM BCP, scaffold E) was used as a positive control for in vivo bone formation to confirm the osteogenic potential of the cells used [22].
The actual porosity of the prepared scaffolds is higher than expected from the used salt volume, and the compression modulus decreases significantly with increasing porosity. The scaffolds (dry, prior to gas plasma treatment and cell seeding) were characterized using micro computed tomography (μ-CT). Relevant parameters like scaffold porosity and pore size distribution were also calculated. As shown in Table 1, the porosity obtained from μ-CT matches the values determined by density measurements quite well. Pore size distributions are given in Fig. 2A. The pore size distributions of the 1000PEOT70PBT30 scaffolds A, B and C with pore sizes up to 648 μm are comparable, as was expected for scaffolds prepared with salt of the same particle size. The pore size distributions of PDLLA (D) (pore sizes up to 760 μm) and BCP (E) (pore sizes up to 1000 μm) are noticeably different from the 1000PEOT70PBT30 scaffolds. The average pore sizes of the 1000PEOT70PBT30 scaffolds (318, 342 and 311 μm for the scaffolds A, B, and C respectively) and the PDLLA scaffold (407 μm) are smaller than the size of the salt particles used (425–500 μm).
(A) Pore size distributions and average pore sizes derived from μ-CT. Scaffolds A, B, C and D were prepared using salt crystals of 425–500 μm. Scaffold F is identical to scaffold A; (B) Accessible pore volume (as a fraction of the total volume) versus sphere diameter (d) for unseeded and dry scaffolds A–E. Notice the lower accessible pore volume of scaffold A; (C)
Accessible surface area (normalized for the total volume) versus sphere diameter (d) for unseeded and dry scaffolds A–E
The accessible pore volume of the scaffolds was determined using μ-CT data. The resulting graphs of the accessible pore volume (given as a fraction of the total volume) versus the sphere diameter (d) as used in the algorithm are shown in Fig. 2B. Even though the average pore sizes and pore size distributions are comparable, there are considerable differences in accessible pore volume of the 1000PEOT70PBT30 scaffolds.
The curve for scaffold A shows that there is much less accessible pore volume at sphere diameters below 300 μm than for the 1000PEOT70PBT30 scaffolds B and C and PDLLA scaffold D. The accessible pore volume of the 1000PEOT70PBT30 scaffolds B and C is quite comparable, showing that the increase in scaffold porosity of 80.6–85.0% does not result in a larger accessible pore volume. For all sphere diameters the accessible pore volume of PDLLA scaffold D is higher than (or equal to) the accessible pore volume of the 1000PEOT70PBT30 scaffolds.
With increasing porosity one would expect a larger surface area available for the BMSCs to attach to and proliferate on, although this area can depend on pore geometry, pore size and size distribution and on pore interconnectivity. For simulated sphere diameters up to 100 μm 1000PEOT70PBT30 scaffold C of 85.0% porosity indeed has a higher accessible surface area than scaffold B of 80.6% porosity (Fig. 2C). Surprisingly scaffold A with the lowest porosity of 73.5% shows the highest accessible surface area (for simulated spheres with a diameter (d) up to 100 μm) and PDLLA scaffold D of 83.5% porosity shows the lowest accessible surface area of the polymeric scaffolds. Here pore size is important too, as for comparable porosities, a smaller pore size will result in a larger available surface area.
Rat BMSCs were seeded on the scaffolds (static seeding, 2 × 105 cells/scaffold) and cultured for 7 d in an osteogenic medium. To estimate the number of cells present on and in the different cell-seeded and cultured scaffolds, the DNA was fluorimetrically quantified using CyQuant® dye. The results are presented in Fig. 3.
Fluorimetric quantification of DNA in the scaffolds after 7 d of culture in an osteogenic medium of rat BMSC culture (triplicate measurements). *: Value significantly different from the other 5 scaffolds. A: 1000PEOT70PBT30, 73.5% porous, average pore size 318 μm; B: 1000PEOT70PBT30, 80.6% porous, 342 μm; C: 1000PEOT70PBT30, 85.0% porous, 311 μm; D: PDLLA, 83.5% porous, 407 μm; E: BCP, 29% porous, average pore size: 837 μm; F: 1000PEOT70PBT30, 73.5% porous, untreated
The higher amounts of DNA present in scaffold C (85.0% porosity), as compared to scaffold B (80.6% porosity), reflect the increase in surface area available for the cells with increasing porosity. Scaffold A shows the smallest amount of DNA even though μ-CT data indicates that this scaffold has the largest accessible surface area (for sphere diameters smaller than 100 μm). After 7 d of culture both the PDLLA and BCP scaffolds (D and E) contain a significantly lower amount of DNA (and hence cells) than the gas plasma treated 1000PEOT70PBT30 A, B and C scaffolds. This is likely due to a limited accessible surface area.
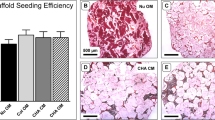
Following the subcutaneous implantation in nude mice to induce ectopic bone formation, the scaffolds were explanted, microtomed and stained using methylene blue and basic fuchsin. This allows differentiating between formed bone, bone marrow, cartilage and fibrous tissues [23]. The amounts of the different tissues formed were determined in middle cross sections of the different scaffolds (implanted in 5-fold) and averaged. The porosity (area corresponding to pores) and the amounts (relative areas) of the different formed tissues (normalized for the porosity) are shown in Table 2. Cross-sections taken from the middle of the scaffolds are shown in Fig. 4.
Representative cross sections of scaffolds, seeded with rat BMSCs and cultured for 7 d, after 4 wks of subcutaneous implantation. Sections shown were taken from the middle of the scaffolds and stained using methylene blue and basic fuchsin. 1000PEOT70PBT30 stains pink, whereas PDLLA appears transparent. All scaffolds (except F) show bone (dark red) and bone marrow (blue/gray) formation. Fibrous tissue and wound exudate stain pink. All scaffolds (except F) show bone and bone marrow formation. In addition scaffold A (1000PEOT70PBT30, porosity 73.5%) shows the formation of cartilage (purple)
When comparing scaffold A, B and C not much difference is observed in terms of the amount of bone and bone marrow tissue formed (see Table 2). Several scaffold C implants were severely distorted upon explantation (data not shown) and it seems that their rigidity is not adequate for use under these conditions. Scaffold E (BCP) clearly shows the ability of the used BMSCs to induce ectopic bone formation upon implantation, as bone and bone marrow are abundantly present on the surface of the scaffold. The negative control scaffold F (untreated 1000PEOT70PBT30, seeded with rat BMSCs and cultured for 7 d in an osteogenic medium) does not show any formation of bone or bone marrow.
The relative amounts of ectopic bone formed in the four different cell-seeded polymeric scaffolds are comparable and vary from 7 to 9%. All 1000PEOT70PBT30, PDLLA and BCP scaffolds show bone marrow formation. The PDLLA scaffold contained considerably more bone marrow than the 1000PEOT70PBT30 scaffolds, even though at the time of implantation there was significantly less DNA (and hence cells) present in the PDLLA scaffold. Besides bone and bone marrow, fairly high amounts of fibrous tissue were observed in many of the scaffolds. In line with previous work [17, 23], the observation of ectopic bone formation in the 1000PEOT70PBT30, PDLLA and BCP porous structures again shows their potential as scaffold materials for the tissue engineering of bone.
Surprisingly, scaffold A (porosity 73.5%) also showed cartilage formation besides bone and bone marrow. The scaffold stiffness can greatly affect cartilage formation in vivo [24, 25]. Although the effect of scaffold stiffness on cartilage formation cannot be excluded, there is another more likely explanation for the observation of cartilage formation. From the histological sections it appears that the pores in which cartilage formation takes place are not well connected (Fig. 5). Cartilage formation is known to be favored by conditions in which the oxygen supply is limited [26]. Implantation of demineralized teeth resulted in cartilage formation when one side was left open and in bone formation when both sides where left open. A comparable observation was made after implantation of hydroxyapatite with channel-like pores, where cartilage was observed in the central zones of the pores [27]. Vascularization is needed for adequate oxygen and nutrient supply and is therefore believed to be of great importance for bone formation [28]. Vascularization of tissue within a scaffold with large pores but with small pore interconnections may be difficult [29].
The poor accessibility of this pore structure also follows from μ-CT. Rat BMSC seeding on the 1000PEOT70PBT30 scaffold A also shows considerably less ingrowth of fibrous tissue and contains much more wound exudate upon implantation than when using the other scaffolds.
Conclusions
After culturing in an osteogenic medium, the BMSC seeded polymer scaffolds described are suited for bone tissue engineering. All implanted cell-seeded scaffolds show bone and bone marrow formation. By varying the scaffold porosity of 1000PEOT70PBT30 scaffolds it was possible to obtain scaffolds with comparable pore size distributions, but with considerable differences in accessible pore volume. However, no significant differences between the different 1000PEOT70PBT30 scaffolds were observed in terms of ectopically formed bone (7–9%) and bone marrow (6–11%). Surprisingly, 1000PEOT70PBT30 scaffolds with a porosity of 73.5% showed cartilage formation. Although the effect of scaffold stiffness cannot be excluded, cartilage formation is most likely due to a poorly accessible pore network.
BMSC seeded PDLLA and BCP scaffolds showed considerably more bone and bone marrow formation and less fibrous tissue in-growth than the 1000PEOT70PBT30 scaffolds, although both scaffolds contained much less cells (as determined by a DNA assay) upon implantation. The scaffold material can be of great influence. In more hydrophobic and rigid scaffolds like PDLLA or BCP, the accessibility of the pore structure is more likely to be preserved under the prevailing physiological conditions than in hydrophilic 1000PEOT70PBT30 scaffolds. When compared to 1000PEOT70PBT30 scaffolds, the PDLLA and BCP scaffolds seem more suited for application in bone tissue engineering. Further improvements, such as adjustment of the PEOT/PBT composition in the polymer used for the scaffolds, are necessary to increase the amount of bone and bone marrow formed in the scaffolds.
References
C. J. DAMIEN and J. R. PARSONS, J. Appl. Biomater. 2 (1991) 187
K. J. L. BURG, S. PORTER and J. F. KELLAM, Biomaterials 21 (2000) 2347
A. A. DESCHAMPS, D. W. GRIJPMA and J. FEIJEN, Polymer 42 (2001) 9335
R. J. B. SAKKERS, J. R. DE WIJN, R. A. J. DALMEYER, R. BRAND and C. A. VAN BLITTERSWIJK, J. Mater. Sci.-Mater. Med. 9 (1998) 375
C. A. VAN BLITTERSWIJK, J. VAN DER BRINK, H. LEENDERS and D. BAKKER, Cell Mater. 3 (1993) 23
C. A. VAN BLITTERSWIJK, D. BAKKER, S. C. HESSELING and H. K. KOERTEN, Biomaterials 12 (1991) 187
A. M. RADDER, H. LEENDERS and C. A. VAN BLITTERSWIJK, Biomaterials 16 (1995) 507
A. M. RADDER, H. LEENDERS and C. A. VAN BLITTERSWIJK, J. Biomed. Mater. Res. 30 (1996) 341
R. VAN DIJKHUIZEN-RADERSMA, S. C. HESSELING, P. E. KAIM, K. DE GROOT and J. M. BEZEMER, Biomaterials 23 (2002) 4719
M. L. C. ANDERSON, W. J. A. DHERT, J. D. DE BRUIJN, R. A. J. DALMEIJER, H. LEENDERS, C. A. VAN BLITTERSWIJK and A. J. VERBOUT, Clin. Orthop. Rel. Res. (1999) 231
M. ROESSLER, A. WILKE, P. GRISS and H. KIENAPFEL, J. Biomed. Mater. Res. 53 (2000) 167
R. CANCEDDA, B. DOZIN, P. GIANNONI and R. QUARTO, Matrix Biol. 22 (2003) 81
C. MANIATOPOULOS, J. SODEK and A. H. MELCHER, Cell Tissue Res. 254 (1988) 317
S. J. PETER, C. R. LIANG, D. J. KIM, M. S. WIDMER and A. G. MIKOS, J. Cell. Biochem. 71 (1998) 55
P. S. LEBOY, J. N. BERESFORD, C. DEVLIN and M. E. OWEN, J. Cell. Physiol. 146 (1991) 370
D. J. RICKARD, T. A. SULLIVAN, B. J. SHENKER, P. S. LEBOY and I. KAZHDAN, Dev. Biol. 161 (1994) 218
S. C. MENDES, M. SLEIJSTER, A. VAN DEN MUYSENBERG, J. D. DE BRUIJN and C. A. VAN BLITTERSWIJK, J. Mater. Sci.-Mater. Med. 13 (2002) 575
S. L. ISHAUG-RILEY, G. M. CRANE, A. GURLEK, M. J. MILLER, A. W. YASKO, M. J. YASZEMSKI and A. G. MIKOS, J. Biomed. Mater. Res. 36 (1997) 1
A. A. DESCHAMPS, M. B. CLAASE, W. J. SLEIJSTER, J. D. DE BRUIJN, D. W. GRIJPMA and J. FEIJEN, J. Control. Release. 78 (2002) 175
M. B. OLDE RIEKERINK, M. B. CLAASE, G. H. M. ENGBERS, D. W. GRIJPMA and J. FEIJEN, J. Biomed. Mater. Res. 65A (2003) 417
Q. P. HOU, D. W. GRIJPMA and J. FEIJEN, Biomaterials 24 (2003) 1937
H. P. YUAN, M. VAN DEN DOEL, S. H. LI, C. A. VAN BLITTERSWIJK, K. DE GROOT and J. D. DE BRUIJN, J. Mater. Sci.-Mater. Med. 13 (2002) 1271
S. C. MENDES, J. BEZEMER, M. B. CLAASE, D. W. GRIJPMA, G. BELLIA, F. DEGLI-INNOCIENTI, R. L. REIS, K. DE GROOT, C. A. VAN BLITTERSWIJK and J. D. DE BRUIJN, Tissue Eng. 9 (2003) S91
J. H. DE GROOT, F. M. ZIJLSTRA, H. W. KUIPERS, A. J. PENNINGS, J. KLOMPMAKER, R. P. H. VETH and H. W. B. JANSEN, Biomaterials 18 (1997) 613
T. G. VAN TIENEN, R. HEIJKANTS, P. BUMA, J. H. DE GROOT, A. J. PENNINGS and R. P. H. VETH, Biomaterials 23 (2002) 1731
C. A. L. BASSETT and I. HERMANN, Nature 190 (1961) 460
Q. M. JIN, H. TAKITA, T. KOHGO, K. ATSUMI, H. ITOH and Y. KUBOKI, J. Biomed. Mater. Res. 51 (2000) 491
J. MAHMOOD, H. TAKITA, Y. OJIMA, M. KOBAYASHI, T. KOHGO and Y. KUBOKI, J. Biochem. (Tokyo). 129 (2001) 163
J. T. SCHANTZ, D. W. HUTMACHER, H. CHIM, K. W. NG, T. C. LIM and S. H. TEOH, Cell Transplant. 11 (2002) 125
Acknowledgments
This study was financed by the European Community (Brite-Euram project BE97-4612). We would like to thank Dr. A. Laib (Scanco Medical AG, Bassersdorf, Switzerland) for the μ-CT analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Claase, M.B., de Bruijn, J.D., Grijpma, D.W. et al. Ectopic bone formation in cell-seeded poly(ethylene oxide)/poly(butylene terephthalate) copolymer scaffolds of varying porosity. J Mater Sci: Mater Med 18, 1299–1307 (2007). https://doi.org/10.1007/s10856-006-0077-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-006-0077-y