Abstract
Herein, we report, for the first time, the influence of the state of spin of the Cr3+ ions on the structure and magnetic behavior of LaFe1−xCrxO3 (0.0 < x < 0.5) perovskite crystal structures to improve their magnetic properties for memory storage and magneto-optical application potential. A series of LaFe1−xCrxO3 (0.0 < x < 0.5) nanocrystals was prepared using the citrate-nitrate auto-combustion route. The influence of the Cr3+ ions on the structure and morphology of the LaFe1−xCrxO3 nanostructures was emphasized using X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), the selected area of electron diffraction (SAED), and scanning electron microscopy (SEM). The results revealed that the inclusion of the Cr3+ ions into the Fe3+ sites leads to an increase in the crystallite size of the LaFe1−xCrxO3 (0.0 < x < 0.5) nanocrystals, from 16.12 to 57.29 nm, to preserve their orthorhombic crystal symmetry. The phase stability and the electron density mapping of the prepared orthoferrite nanocrystals were verified using the Goldschmidt tolerance factor and Rietveld refinement. The magnetic properties were evaluated and discussed based on Kanamori–Goodenough (KG) regulations and Heisenberg Hamiltonian notation. The results showed that the inclusion of the 20 mol% of Cr3+ ions in the Fe sites results in an enhancement of the magnetic parameters. The enhancement of the magnetic behavior was argued to the tendency of the Cr3+ ions to be in the high spin state when included in the Fe3+ ions site. This improvement in the magnetic performance of the LaFe1−xCrxO3 (x = 0.2) nanocrystals will open a new avenue for using this nanomagnetic material in the fields of memory storage and magneto-optical devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare-earth orthoferrites with a typical formula of RFe1−x MxO3 with (R = Pr, Nd, Dy, La, Eu, Gd, Tb, Sm, Er, etc.) and (M = transition metals as Mn, Cr, Zn, etc.). These rare-earth elements make a significant contribution to the ferrites’ net magnetic response. These classes of metal oxide nanoparticles have attracted great attention due to their multi-functionalities and their significant magnetic properties [1,2,3,4,5]. As a result, these perovskites have promising characteristics for applications such as gas separation, memory storage devices, fuel cells, gas sensing, magneto-optical devices, and electromagnetic equipment, as well as modern electronic technology [6,7,8,9,10]. It is commonly recognized that combining several compositions with superior electrical properties results in new composite materials, which have grabbed the curiosity of researchers in recent years. The addition of a second phase can significantly enhance the resulting composite material’s electrical characteristics [11, 12].
LaFeO3 (LFO), one of the key rare-earth orthoferrites, has been widely reported for a broad range of industrial applications, including solid oxide fuel cells, sensors, and environmental catalysts [13], among others. LFO is a rare-earth orthoferrite that crystallizes in an orthorhombic structure with pbnm (No.62) symmetry [14], with a great value of Neel temperature of 750 K, and an antiferromagnetic (AFM) axis oriented along the crystallographic a-axis [9, 10, 15]. As K. Rachid et al. [1] point out, the synthesis process used has a considerable influence on characteristics and the composition’s structure. Many chemical methods have been used to synthesize LFO and related perovskite compositions, including sol–gel, hydrothermal, microwave-assisted, co-precipitation, citrate-gel auto-combustion approach, mechanochemical synthesis [16,17,18,19,20,21,22,23].
First, A. Paul Selvadurai et al. [15] demonstrated the LFO multiferroic characteristics, studied the interaction of (AFM/FM), and reported irreversibility in the FCC–ZFC curve. Second, according to the Goodenough-Kanamori hypothesis, Cr3+ is the optimum substitute element for increasing the magnetization of LaFeO3 since Fe3+ and Cr3+ arranged at the octahedral site as Fe3+(d5)–O2−–Cr3+(d3) would have improved the magnetism due to the super exchange interaction [24]. Yan Chen et al. discovered that substituting Cr3+ ions for Fe3+ ions increase the proportional fraction of Fe3+–O2−–Cr3+ FM super exchange interaction, resulting in a maximum magnetic moment at x = 0.5 in their research of magnetic characteristics of LaFe1−xCrxO3 (0 > x > 1). Even though iron and chromium ions were being randomly allocated at the Fe site, an improved saturation magnetization of 0.21 B/f.u [25] was observed and reported later [26,27,28].
Y.M. Abbas et al. used the sol–gel auto-combustion route to synthesize the orthoferrite system La1−xYxFeO3, which exhibits a canted antiferromagnet and shows a weak ferromagnetism at 300 K [29], and they argued that the contribution of magnetic attitude of the synthesized compositions reflected differences in magnetic moments of Fe3+ (5 µB) and Fe+ 2 ions (4 µB). With rising Y3+-content, the effective moment showed a significant decrease.
As a result, Cr3+ substitution in LaFe1−xCrxO3 could result in intriguing magnetic and electrical properties. The layered development of LaCrO3–LaFeO3 using the molecular beam epitaxy (MBE) approach corroborated the increase in magnetization [30].
Herein, we report for the first time the influence of the state of spin of the Cr3+ ions on the structure and magnetic behavior of LaFe1−xCrxO3 (0.1 < x < 0.5) perovskite crystal structures. The inclusion of the Cr3+ ions in the Fe sites results in a dramatic change in the Goldschmidt tolerance factor and a change in the dimensions of the formed crystallites. This change gave rise to an enhancement of the magnetic parameters. The enhancement of the magnetic behavior was argued to the tendency of the Cr3+ ions to be in the high spin state when included in the Fe3+ ions site.
2 Experimental details
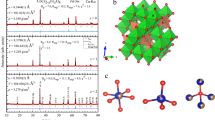
Numerous methods were used for synthesis of orthoferrites, including sol–gel, hydrothermal, microwave-assisted, co-precipitation, citrate-gel auto-combustion approach, and mechanochemical synthesis [16,17,18,19,20,21,22,23]. The orthoferrite system LaFe1−xCrxO3 with (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) were synthesized via sol–gel auto-combustion route, and citrate acid is used as a fuel. Various steps were used as described in Fig. 1. The starting raw materials for synthesis are La(NO3)3.6H2O (Alfa Aesar, purity 99.99%), Fe(NO3)3.9H2O (Alfa Aesar, purity 99.98%), Cr(NO3)3.9H2O (Alfa Aesar, purity 99.90%), and citric acid monohydrate C6H8O7.H2O (Alfa Aesar, purity 99.50%). Separately, one hour was spent dissolving the metal nitrates in distilled water before they were mixed stoichiometrically with constant stirring. The ratio of nitrates to fuel was kept constant (1:1). A temperature range of 353–363 K was used to dissolve the solution while being stirred continuously at 1700 rpm, and the pH was regulated by adding the right quantity of (NH3.OH 33.3%) aqueous solution drop by drop during the stirring operation to raise the PH to ~ 7. The dried gel was decomposed into the powder at 573 K in an air environment, and then a vigorous thermochemical combustion process produced the deep brown powder. By increasing the amount of chromium, the blackness of the powder rose. The resulting power perovskites were grinded in an agate mortar and calcinated in open air at 1123 K for 6 h.
All the synthesized perovskites were investigated by (XRD, X’Pert pro PANalytical MRD instrument) using Cu-Kα irradiation (λ = 1.54056 Å) for phase identification and structure. The Rietveld approach was employed to determine the refined crystallographic parameters such as unit cell dimensions, electron density, breadth, atomic coordinates, thermal parameters, microstructural parameters, atomic site occupancies, etc. for each perovskite. The FullProf package was used to perform the structural refinement of XRD patterns for the prepared samples. The refinement process was performed on the patterns with a 2° (2θ)/min counting rate per step. The refining approaches for all the perovskites under study began with crystallographic data from LFO [31]. In the structural analysis, the peak shapes for distinct samples were illustrated using an asymmetric “Thompson-Cox-Hastings Pseudo-Voigt function with axial divergence symmetry”, the size/strain broadening caused by the sample contribution might be calculated based on this. Using the GFourier tool (FullProf Suite Package), the electron density mapping (EDM) within the unit cell was estimated and visualized. Several literary works [32,33,34,35,36,37,38,39] have detailed descriptions of the mathematical methodologies used in the Rietveld approach. The crystallite size and shape of the calcinated powder were investigated using HRTEM, JEOI-JEM 2100 equipment (National Research Center (NRC), Egypt). The materials’ morphology and composition analysis were studied using Quanta FEG-250 SEM-EDX. Using a VSM, the magnetization (M) was measured over a magnetic field (H) range of (0–20kOe) (Lakeshore 7310, ACR, El-Dokki, Egypt).
3 Result and discussion
3.1 Structure analysis
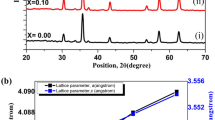
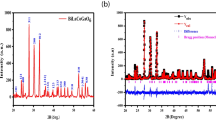
The crystallography of the prepared samples was evaluated using conventional XRD. Figure 2 displays the XRD spectrum for the LaFe1−xCrxO3 perovskite group. The diffraction patterns were refined using Rietveld approach, which yielded peaks for the (101), (121), (220), (202), (240), (242), and (204) planes, correlated with (JCPDS: No. 88–0641), of which a negative x/a site coordinate value yields from the symmetric evolution of Pnma to Pbnm [40], The XRD measurements and Rietveld analysis confirmed that there are no secondary phases that have been formed. It also confirms that the prepared compositions formed a monophase inside the detection limit of XRD. According to Selvadurai et al. [15], the shift of diffraction peaks to higher 2θ with increasing Cr doping is attributable to the less significant ionic radius of the Cr+ 3 cation compared to the Fa+ 3, which they attribute to the absence of any secondary phases in the starting materials [13].
The Goldschmidt’s tolerance factor is an indicator for crystal structure stability and deformation. Goldschmidt tolerance factor (t) of the perovskites LaFe1−xCrxO3 is a dimensionless quantity computed using the ionic radii ratio and is expressed as follows [3, 41]:
where \({\text{R}}_{\text{La}}\), \({\text{R}}_{\text{C}\text{r}}\), \({\text{R}}_{\text{O}}\), and \({\text{R}}_{\text{Fe}}\) are the ionic radii of the La, Cr, O and Fe ions, sequentially.
The tolerance factor (t) is a geometric factor describing the size difference that occurs when the cations of A-site are too small to occupy the space in the 3-dimensional octahedral [BO6] network. If the A-site cation is less than the ideal value, t will be less than unity.
Therefore, in attempting to occupy space, the [BO6] octahedra will tilt. For an ideal cubic structure (t = 1), the perovskite structure is steady when (0.75 < t < 1.0) and cubic when (t > 0.95) [42]. The orthorhombic symmetry of the perovskites is assured by (t) values in the range of (0.85 < t < 0.95) [3, 43]. Lanthanum and chromium ions have ionic radii of (1.216 Å) and (0.615 Å) on a 9-fold and 6-fold coordinations, respectively. On a 6-fold symmetry of octahedral [FeO6], iron and oxygen have values of (0.645 Å) and (1.400 Å), respectively. Because of the variation of both Fe+ 3 and Cr+ 3 ionic radii, which causes the reduction in the cell volume. The (t) values increase with rising chromium concentration at the expense of iron ions, as displayed in Fig. 3. The increase in deformation was caused by tilting of the octahedron [FeO6] due to a decrease in RB cation size. So, when t < 1, the Fe–O–Fe ≠ 180° change in the out of plane Fe–O bonds, which gives rise to the tilting mode [26, 30].
Figure 4a–f shows the Rietveld refinement of XRD patterns for the perovskite system LaFe1−xCrxO3. We refined the lattice parameters for all the compositions, and Table 1 summarizes the results. Since the Cr3+ cation is smaller than the Fe3+ cation, the substitution with Cr results in a reduction of cell volume V, which is an expected result. As the chromium substitution increases, the lattice parameters a, b, and c fall slightly, suggesting a considerable decrease in system volume, which causes lattice geometry distortion. Figure 5 illustrates the variation of cell volume (V) and grain size (D) as a function of Cr concentration. The lattice parameters of the prepared perovskites coincide with previously published values [25, 44]. The decreases in crystallite size with increasing the amount of Cr3+ ions may be attributed to the substitution of Fe3+ ions with large ionic radius by the Cr3+ ions with small ionic radius and/or the inclusion of Cr3+ ions may cause the formation of oxygen vacancies. Both of these two reasons give rise to the decrease in crystallite size [44, 45].
For the perovskites under investigation, the particle size (DXRD), and micro-strain were determined using the Williamson–Hall (W–H) plot, and the estimated particle size values are listed in Table 1. Equation 3 correlates strain-induced broadening caused by crystal defects and distortion [29].
According to the several methodologies, the overall integral breadth of a Bragg peak is made up of the additive components of the crystallite size and micro-strain broadening. The distinction of size and strain broadening in the (W–H) analysis was based on the various θ dependencies of both effects, as shown in the following equations [29]:
All of the material’s properties should be very independent of the direction in which they are examined because the strain is expected to be isotropic. For the refined diffraction data of LaFe1−xCrxO3 powder, the term (βhkl cosθ) was drawn as a function of (4sinθ). W–H plots for the refined XRD data show the strain term computed from the slope (ɛ) of the fitted line and the size part calculated from the line cutoff (Kλ/D) as presented in Fig. 6. Based on the W–H plot and Rietveld refinement of the XRD data for the prepared perovskites, Table 1 displays the calculated mean particle size, which ranged from 16.2 to 57.29 nm. A GFourier (FullProf software package) is employed to compute and display the electron density \(\rho\)(\(\text{x},\text{y},\text{z}\)) as a result of incident X-ray scattering on the unit cell. These estimations use the Fourier method and the fast Fourier transform (FFT). Based on the FFT in the fitted XRD data, Eq. 6 is used to estimate the electron density (ED) [29].
where \(\rho (\text{x},\text{y},\text{z})\) is the electron density at a position \(\text{x},\text{y},\text{z}\) in a unit cell volume V, \(\left|{\text{F}}_{\left(\text{h}\text{k}\text{l}\right)}\right|\) is the structure factor magnitude, and \({\alpha }_{\left(hkl\right)}\) corresponds to the phase angle of each Bragg peak.
Figure 7 shows 3D maps for perovskites LaFe1−xCrxO3 with (x = 0.0, 0.2, and 0.5). Thicker ED contours indicate the existence of a heavy element among the unit cell’s fundamental elements of the prepared orthoferrites. In contrast to the colored areas, which range in color from red to violet-brown, the contour line of zero-level density is shown in black, depicting rising ED levels around the La cation. The positions of the several oxygen atoms in the unit cell show relatively substantial displacements compared to the FelCr and La positions, where significant positive peaks belong to the 4c positions.
The cations FelCr and La are positioned at (0.0, 0.0, 0.5) and (0.028, 0.250, 0.993), respectively, according to the Rietveld refinement results. Figure 7 clearly illustrates the variation in scattering density between the O2− anion, La, and FelCr cations. We shall be able to understand the magnetic properties of the multiferroic system due to this variability in the ED in the crystal lattice.
3.2 TEM analysis
HRTEM imaging of the structure was performed to provide additional support for the formation of nanoparticles, such as particle shape and size for perovskite LaFe1−xCrxO3 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) nanocrystallites. According to XRD results, the single-phase nanocrystalline material, as revealed in Fig. 2, is composed of many tiny single crystals.
As displayed in Fig. 8, HRTEM images confirmed that the particles were an agglomeration of several quasi-spherical particles that had quietly accumulated, and the single-direction configuration of the lattice fringes provided further evidence of the perovskites’ polycrystalline nature. In TEM images, particle agglomeration is responsible for the distinct values of crystallite size obtained by TEM and XRD. The estimation of separate, unagglomerated nanoparticles (NPs) is simple. When NPs are attached together or have an irregular shape, the precise size distribution can be more difficult to measure. The mean particle size calculated from TEM micrographs was approximately 57 nm for LaFeO3 and decreased to 17 nm for LaFe0.6Cr0.4O3, which is very well consistent with XRD results and W–H calculations. According to the HRTEM image of the NPs-suggested part, lattice planes (101) and (121), respectively, have interplanar spacings of 0.39 and 0.27 nm for samples LaFeO3 and LaFe0.7Cr0.3O3, as seen in Fig. 9 and verifying the XRD results. The main diffraction zones correspond to (101), (121), (220), (202), (242), and (204) of orthorhombic nanocrystalline orthoferrites (SG: Pnma), with no impure phase spots, and agree with XRD results. The Selected Area Electron Diffraction (SAED) patterns of perovskite LaFe0.5Cr0.5O3 were indexed using the CSpot software, as illustrated in Fig. 10. Table 2 summarizes and compares the values of such interplanar spacing in sample LaFe0.5Cr0.5O3 as revealed by XRD and TEM investigations. The intensity of the rings was related to the number of diffracting particles, which was determined by the size of the selected area. For polycrystalline materials, large selected areas are necessary to obtain good diffraction patterns.
3.3 SEM-EDX characterization
Scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM/EDX) was used to examine the surface morphologies of the calcinated samples. The SEM images clearly show the formation of agglomerated, nonuniform spherical grains. As shown in Fig. 11, raising the Cr substitution reduces grain size and increases the agglomeration of NPs.
As shown in Fig. 11, the particle configurations of perovskites LaFe0.7Cr0.3O3 and LaFe0.5Cr0.5O3 are almost the same and likely to become spherical after 5 h of calcination at 800 oC, compared with the as-prepared LaFeO3, these findings suggest that LaFe1−xCrxO3 nanostructures’ morphology is greatly influenced by the calcination temperature. The mean grain diameter was approximately 57 nm for pure LFO and reduced to 37.4 nm for LaFe0.5Cr0.5O3. Figure 11a–c illustrates an EDX elemental analysis of the perovskites LaFe1−xCrxO3 (x = 0.0, 0.3, and 0.5). All peaks are assigned to the spectrum arising from the Fe, La, and O elements of the prepared perovskites. This implies the high purity of the prepared samples. The concentrations of these elements are presented in tables set in Fig. 11a–c. It is noted that the concentrations of these elements agree with the calculated amounts of the elements during preparation, confirming the stoichiometry of the prepared samples owing to the quality of the synthetic recipe compared to the other methods [46]. It is also noted that the inclusion of Cr3+ ions into Fe sites leads to a slight decrease of the oxygen amount, implying that there is the possibility of the formation of oxygen vacancies [47].
3.4 Magnetic measurements
Based on the M(H) curves, the magnetic parameters were calculated, including saturation magnetization (Ms), remnant magnetization (Mr), retentivity, and coercive field (Hc). The magnetic hysteresis over the M(H) loop for all the synthesized compositions was characterized at 300 K by using VSM in a field range of ± 20 kOe, as illustrated in Fig. 12. The magnetic susceptibility (χ) measurements of the prepared powder were determined by a VSM inside a field of 1.55 kOe from 400 to 900 K. The hysteresis behavior of samples, as shown in Fig. 12, indicates a weak FM, and this could be due to many reasons. The first possible reason may be due to the oxygen vacancy effect, which may decrease the exchange interaction. These oxygen vacancies show a negative indirect exchange according to Kanamori–Goodenough (KG) empirical rule [48]. Therefore, these oxygen vacancies may lead to the formation of a weak magnetic state, such as spin glass [49, 50].
The superexchange (SE) interaction is the second reason. In accordance with Kanamori–Goodenough (KG) regulations, Fe3+–O2-–Fe3+ and Cr3+–O2-–Cr3+ SE interactions are antiferromagnetic [51, 24], whereas Cr3+–O2-–Fe3+ is expected to be ferromagnetic. Previously, there has been no evidence of strong FM in bulk form of Cr-doped LaFe1−xCrxO3, as noted in LaCrO3–LaFeO3 layered growth [30]. Because Cr substitutions prefer the Cr–Cr coupling on substitution in the host metal cation, as well as the variation of Cr cation in the host site, showing up a weak FM [52]. These Cr3+–Cr3+ coupling behaviors were detected in LaFe0.8Cr0.2O3 and LaFe0.7Cr0.3O3. The third reason for weak FM behavior could be attributable to a cluster or an impurity phase coexisting with the first two reasons [53]. As a result, the room-temperature FM in the perovskites under investigation can be explained by the SE interaction of Fe3+–O2-–Fe3+ ions, which results in the FM shell model at a particle’s surface because of irregular spins and the core of AFM particles as a result of ordering spins [54, 55]. With decreasing crystallite size and the high surface area of these Fe3+ ions, there was an increase in the disordered spin volume fraction relative to the ordered spin volume fraction. In addition, this attitude has also been discovered in other types of perovskites. A. Paul Blessington Selvadurai et al. [15] reported a value close to the Mr and Hc of our samples with (x = 0.0, 0.1), because of the well-grown nanocrystallites, surface disorder is reduced caused by uncompensated spins, while samples LaFe0.8Cr0.2O3 and LaFe0.7Cr0.3O3 show a very small Hc of 75.5 and 28.1 Oe, respectively, because of surface oxide formation and exchange coupling/bias at the resulting interface might be the reason for that drop [27, 54]. Table 3 provides a summary of the magnetic parameters attained from the room-temperature magnetic hysteresis loops for whole compositions. Figure 13 shows that the Hc value gets reduced until LaFe0.7Cr0.3O3, and this behavior may be attributed to cluster spin [15]. The position of the Fe+ 3 and Cr+ 3 ions, where the Fe+ 3 ions are present in a mostly B-site, is where the deviation from the ideal perovskite is most apparent. Because the ordering of Fe moments is somewhat canted rather than precisely antiparallel, there is little net magnetization as a result, which leads to the weak FM that we have previously described [37, 52]. The tilting that decreases a net magnetic moment perpendicular to the uniaxial magneto-crystalline anisotropy is induced by the Dzyaloshinsky-Moriya antisymmetric exchange. The orthoferrite structure exhibits some distortion, as previously noted by C. Gökhan Ünlü et al. [34], which is attributed to the tilts of the octahedral units [FeO6] around the b and c axes, as seen in Fig. 14. This distortion affects the AFM ordering between Fe3+ ions and results in a considerable reduction of the ideal bond angle of 180o. Weak FM behavior appears to be a result of the alignment of the Fe3+ moments being somewhat canted and the LFO structure exhibiting net magnetization [34].
The variation in the perovskites’ reciprocal molar magnetic susceptibility (1/χM) with absolute temperature in a 1.55 KOe applied field is illustrated in Fig. 15 for LaFe1−xCrxO3, with (x = 0.0, 0.2. 0.4, and 0.5) at a temperature range of (400–900 K). It is obvious that as temperatures rise, the values of 1/χM rise as well. It is conceivable for some antiferromagnetic phases to exist as well as for local magnetic phase separation to cause a reduction in susceptibility. The curves 1/χM(T) for samples under investigation were examined using the Cuire–Weiss formula [56]. The susceptibility of FM materials given according to Eq. 7:
where C = NAµB2 µeff2/3kB is the Curie constant, NA is Avogadro number, µB is the Bohr magneton, \({\mu }_{eff}={\mu }_{B} g \sqrt{S(S+1)}\) is the effective magnetic moment, g=2 is the gyromagnetic ratio, and S is the magnetic spin, kB is Boltzmann constant, and θ is the paramagnetic Weiss temperature. We determined θ from the intercept of the straight line with the temperature axis for the set of χM−1(T) curves, where the positive θ values pointing to the FM nature of the prepared samples, as listed in Table 4. One could suggest that a superparamagnetic (SP) behavior is obtained by comparing the hysteresis loops to the behavior of χM−1 (T). One may then explain this trend by arguing that if Cr+3 ions take the place of Fe+3 ions, two factors occur on the magnetic sublattice of octahedral [FeO6]. The first is that when the Cr substitution is raised, the magnetic lattice is diluted, and the FM arrangement switches from a short-range to a long-range one. With an increase in Cr3+ doping, the FM character is weakened, and after that, the effective magnetic moment (µeff) has been reduced, as displayed in Table 4. The exchange interaction Jex/KB is also reduced by increasing the Cr content, as illustrated in Fig. 16. The second involves lessening the tilting of the octahedra (FeO6) while increasing the Cr substitution in a bid to enhance their ferromagnetism.
On the other hand, the χM(T) and M(T) curves of the samples (x = 0.0, 0.2, 0.4, and 0.5) reveal the weak FM character and a significant drop in χM because of the non-magnetic nature of Cr3+ ions when they replace Fe3+ ions in the crystal lattice of LaFe1−xCrxO3 as shown in Fig. 17a. The Curie temperatures (Tc) for LaFeO3, LaFe0.8Cr0.2O3, LaFe0.6Cr0.4O3, and LaFe0.5Cr0.5O3 were determined based on M(T) graphs, as shown in Fig. 17b. The values of Tc were reduced from approximately 730 K for LaFeO3 to 682 K for LaFe0.5Cr0.5O3. There are two magnetic sublattices in orthoferrites, one for the iron ions Fe3+ and the other for RE (La3+). In our present case, the impact of the magnetic properties is controlled by the Fe3+ sublattice denoted by the octahedron [FeO6], since the La3+ ion has a diamagnetic nature. By increasing the substitution level of Cr3+, the interaction of Cr3+–O2−–Cr3+ is very weak, and the Cr3+ (d3) dilutes the antiferromagnetic (Fe3+–O2−–Fe3+ (d5)) matrix, leading to reduced magnetization.
In FM materials, the magnetizations are parallel aligned as a result of exchange coupling between their nearest neighbors. As explained in Heisenberg Hamiltonian notation, the exchange coupling between spins closest to each other could be expressed as follows [29, 57, 58]:
where Jex is the exchange integral, and Si and Sj are two nearby spins. Whether we have FM or AFM coupling depends on the sign of Jex, which is + ve for ferromagnetic materials. The electrons travel between nearby atoms when the interatomic distance is small; according to Bethe and Slater [59], this increases the antiparallel ordering, and a -ve value of Jex will be obtained (AFM material).
Based on the Weiss field theory [27], Jex for typical perovskites were calculated from Eq. 9, which illustrates the relationship between TC and Jex in the FM case.
where s is the spin (s = 5/2 for Fe3+ ion), Z is the number of molecules in the unit cell. The values of Jex are reported in Table 4.
The total magnetic moment was determined according to the relation [57, 58]:
Since the highest magnetic saturation occurred at x = 0.2; therefore, the µcr+3 was determined based on Eq. 10 and found to be 0.046 µB. This value is very close to the experimental value obtained from M–H loop as shown in Fig. 12. Thus, one may conclude that the increase of the amount of Cr+ 3 ions up to x = 0.2, some of the Cr3+ ions tend to fill the tetrahedral site instead of the octahedral site of orthorhombic perovskites crystal structure.
4 Conclusion
The perovskite samples LaFe1−xCrxO3 (X = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) were synthesized by wet-chemical sol–gel auto-combustion route. All compositions crystalize in orthorhombic structure with Pnma symmetry, without any impurity phases from starting materials, according to a structural investigation using powder XRD analysis. With increasing Cr substitution at Fe site, the cell volume decreases because of the difference in the ionic radii of Fe+ 3 and Cr+ 3 ions. Because of the disparity in ionic radii between Cr+ 3 and Fe+ 3 ions, the tolerance factor demonstrated the prepared compositions’ phase stability and showed a considerable rise with increasing Cr+ 3 content at the expense of Fe+ 3 ions. The mean crystallite size of the produced samples was estimated using the W–H plot based on XRD data, which show variety from 16.12 to 57.29 nm. By using HRTEM micrographs to further investigate the microstructure of all the investigated samples, it was discovered that the particles are quasi-spherical and quite close to the XRD crystallite size. The SAED patterns of the produced orthoferrites revealed spotty ring patterns compatible with the XRD data, indicating the crystallization of samples in orthorhombic structure. The electron density visualization shows an increase in ED around the La3+ ions in 4c sites and relatively substantial displacements in the various oxygen positions in the unit cell. The magnetization of the samples depending on composition and is primarily caused by Fe3+–O2−–Fe3+ and Cr3+–O2−–Cr3+ superexchange AFM interactions, according to the M(H) loops of the perovskite system, showed weak FM behavior, due to the canted spin and uncompensated spins at the surface. The change of state of spin of Cr ions and their migration from octahedral site to tetrahedral site leads to the increase of saturation magnetization up to concentration of 20%. These unique features may enable the prepared perovskites to be employed in the fields of memory storage and magneto-optical devices.
Data availability
All authors declare that the data at the heart of research article are the authors' original data, these data have not received prior publication and is not under consideration for publication elsewhere.
References
M.S. Chavali, M.P. Nikolova, SN Appl. Sci. 1, 607 (2019)
Z. Zhou, L. Guo, H. Yang, L. Qiang, F. Ye, J. Alloys Compd 583, 21–31 (2014)
M.A. Ahmed, S.I. El-Dek, Mater. Sci. Eng., B 128, 30–33 (2006)
A.V. Trukhanov, V.O. Turchenko, I.A. Bobrikov, S.V. Trukhanov, I.S. Kazakevich, A.M. Balagurov, J. Magn. Magn. Mater 393, 253–259 (2015)
M.V. Zdorovets, A.L. Kozlovskiy, D.I. Shlimas, D.B. Borgekov, J. Mater. Sci.: Mater. Electron 32, 16694–16705 (2021)
C. Sasikala, N. Durairaj, I. Baskaran, B. Sathyaseelan, M. Henini, E. Manikandan, J. Alloys Compd 712, 870–877 (2017)
A.A. Saukhimov, M.A. Hobosyan, G.C. Dannangoda, N.N. Zhumabekova, G.A. Almanov, S.E. Kumekov, K.S. Martirosyan, Int. J. Self Propag. High Temp. Synth. 24, 63–71 (2015)
V. Mary Teresita, A. Manikandan, B. Avila Josephine, S. Sujatha, S.A. Antony, J. Supercond. Novel Magn 29, 1691–1701 (2016)
E. Hema, A. Manikandan, P. Karthika, S. Arul Antony, B.R. Venkatraman, J. Supercond. Novel Magn 28, 2539–2552 (2015)
K. Chinnaraj, A. Manikandan, P. Ramu, S. Arul Antony, P. Neeraja, J. Supercond. Novel Magn. 28, 179–190 (2015)
A.L. Kozlovskiy, M.V. Zdorovets, Mater. Chem. Phys 263, 124444 (2021)
M.A. Almessiere, Y. Slimani, N.A. Algarou, M.G. Vakhitov, D.S. Klygach, A. Baykal, T.I. Zubar, S.V. Trukhanov, A.V. Trukhanov, H. Attia, M. Sertkol, I.A. Auwal, Adv. Electron. Mater. 8, 2101124 (2022)
M. Enhessari, A. Salehabadi, A. Khoobi, R. Amiri, Mater. Sci-Pol 35, 368–373 (2017)
M. Etter, M. Müller, M. Hanfland, R.E. Dinnebier, Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 70, 452–458 (2014)
A.P.B. Selvadurai, V. Pazhanivelu, C. Jagadeeshwaran, R. Murugaraj, I.P. Muthuselvam, F.C. Chou, J. Alloys Compd 646, 924–931 (2015)
S. Acharya, A.K. Deb, D. Das, P.K. Chakrabarti. Materials Letters 64, 415–418 (2010)
M. Mesbah, S. Hamedshahraki, S. Ahmadi, M. Sharifi, C.A. Igwegbe, Methods X 7, 100786 (2020)
R.E. El-Shater, HEl. Shimy, S.A. Saafan, M.A. Darwish, D. Zhou, A.V. Trukhanov, S.V. Trukhanov, F. Fakhry, J. Alloy. Compd. 928, 166954 (2022)
X. Niu, H. Li, G. Liu, J. Mol. Catal. A: Chem 232, 89–93 (2005)
F.A. Fabian, P.P. Pedra, J.L.S. Filho, J.G.S. Duque, C.T. Meneses, J. Magn. Magn. Mater 379, 80–83 (2015)
S.V. Trukhanov, I.O. Troyanchuk, M. Hervieu, H. Szymczak, K. Bärner, Phys. Rev. B 66, 184424 (2002)
I.Z. Zhumatayeva, I.E. Kenzhina, A.L. Kozlovskiy, M.V. Zdorovets, J. Mater. Sci.: Mater. Electron 31, 6764–6772 (2020)
D.I. Shlimas, A.L. Kozlovskiy, M.V. Zdorovets, J. Mater. Sci.: Mater. Electron 32, 7410–7422 (2021)
J. Kanamori, J. Phys. Chem. Solids 10, 87–98 (1959)
W. Hu, Y. Chen, H. Yuan, G. Zhang, Li. Guanghua, P. Guang sheng, S. Feng, J. Solid-State Chem 183, 1582–1587 (2010)
D. Sánchez-Rodrígueza, H. Wadaa, S. Yamaguchia, J. Farjasb, H. Yahiro, Ceram. Int. 43, 3156–3165 (2017)
A. Belayachi, E. Loudghuu, M. El Yamani, M. Nogues, J.L. Dormann, M. Taibi, Annales de Chimie - Science des Matériaux 23, 297–300 (1998)
A.K. Azad, A. Mellerga, S.-G. Eriksson, S.A. Ivanov, S.M. Yunus, F. Lindberg, G. Svensson, R. Mathieu, Mater. Res. Bull. 40, 1633–1644 (2005)
Y.M. Abbasa, A.B. Mansour, S.E. Ali, A.H. Ibrahim, J. Magn. Magn. Mater 482, 66–74 (2019)
K. Ueda, H. Tabata, T. Kawai, J. Appl. Phys 89, 2847–2851 (2001)
M. Marezio, P.D. Dernier, Mater. Res. Bull 6, 23–30 (1971)
H.M. Rietveld, Acta Crystallogr. A 22, 151 (1967)
H.M. Rietveld, J. Appl. Crystallogr 2, 65 (1969)
C.G. Ünlü, M.B. Kaynar, T. Şimşek, A. Tekgül, B. Kalkan, Ş Özcan, J. Alloys Compd 784, 1198–1204 (2019)
S. Bid, S.K. Pradhan, J. Appl. Crystallogr 35, 517 (2002)
S. Bid, S.K. Pradhan, Mater. Chem. Phys 82, 27 (2003)
D.A. Vinnik, A.Yu. Starikov, V.E. Zhivulin, K.A. Astapovich, V.A. Turchenko, T.I. Zubar, S.V. Trukhanov, J. Kohout, T. Kmječ, O. Yakovenko, L. Matzui, A.S.B. Sombra, D. Zhou, R.B. Jotania, C. Singh, A.V. Trukhanov, Ceram. Int 47, 17293–17306 (2021)
V.A. Turchenko, S.V. Trukhanov, V.G. Kostishin, F. Damay, F. Porcher, D.S. Klygach, M.G. Vakhitov, L.Yu. Matzui, O.S. Yakovenko, B. Bozzo, I. Fina, M.A. Almessiere, Y. Slimani, A. Baykal, D. Zhou, A.V. Trukhanov, J. Energy Chem. 69, 667–676 (2022)
Y.M. Abbas, A. Bakry, M.A. Hassan Ibrahim, Am. J. Mater. Sci. 4, 84–96 (2014)
J. Prado-Gonjal, A.M. Are valo-Lo pez, E. Moran, Mater. Res. Bull 46, 222–230 (2011)
K. Tezuka, Y. Hinatsu, J. Solid State Chem 141, 404–410 (1998)
R.L. Cook, A.F. Sammells, Solid State Ionics 45, 311–321 (1991)
M.A. Green, A. Ho-Baillie, H.J. Snaith, Nat. Photonics 8(7), 506–514 (2014)
S.V. Trukhanov, L.S. Lobanovski, M.V. Bushinsky, V.A. Khomchenko, V.V. Fedotova, I.O. Troyanchuk, H. Szymczak, Semiconductors 41, 507–511 (2007)
A.L. Kozlovskiy, D.I. Shlimas, M.V. Zdorovets, J. Mater. Sci.: Mater. Electron 32, 12111–12120 (2021)
S.V. Trukhanov, I.O. Troyanchuk, I.M. Fita, H. Szymczak, K. Bärner. J. Magn. Magn. Mater. 237, 276–282 (2001)
A. Kozlovskiy, K. Egizbek, M.V. Zdorovets, M. Ibragimova, A. Shumskaya, A.A. Rogachev, Z.V. Ignatovich, K. Kadyrzhanov, Sensors 20, 4851 (2020)
S.V. Trukhanov, A.V. Trukhanov, H. Szymczak, Low Temp. Phys 37, 465–469 (2011)
S.V. Trukhanov, A.V. Trukhanov, A.N. Vasiliev, A.M. Balagurov, H. Szymczak, J. Exp. Theor. Phys. 113, 819–825 (2011)
H. Ahmadvand, H. Salamati, P. Kameli, A. Poddar, M. Acet, K. Zakeri, J. Phys. D 43(24), 245002 (2010)
B. John, Goodenough Phys. Rev. 100, 564–573 (1955)
L. Capogna, A. Martinelli, M.G. Francesconi, P.G. Radaelli, J. Rodriguez Carvajal, O. Cabeza, M. Ferretti, C. Castellano, T. Corridoni, N. Pompeo, Phys. Rev. B 77, 104438 (2008)
Z. Shifeng Zhao, W. Ma, Y. Xing, A. Ma, Q. Bai, J. Yun, Chen, Thin Solid Films 570, 351–355 (2014)
J. Li, D. Song, T.M. Wang, Z. Su, J. Alloy. Compd 261, 299–303 (1997)
J.W. Seo, E.E. Fullerton, F. Nolting, A. Scholl, J. Fompeyrine, J.P. Locquet, J. Phys. Condens. Matter 20, 264014 (2008)
J.H.V. Vleck, The Theory of Electric and Magnetic Susceptibilities, 1st edn. (Oxford University Press, London, 1932), p. 207
A. Yaresko, V. Antonov, J. Magn. Magn. Mater 310, 1672–1674 (2007)
W.E. Mahmoud, A.A. Al-Ghamdi, F.A. Al-Agel, E. Al-Arfaj, F.S. Shokr, S.A. Al-Gahtany, A. Alshahrie, W. Shirbeeny, L.M. Bronstein, G.W. Beall, Ceram. Int 41(7), 9101–9106 (2015)
W.E. Mahmoud, A.A. Al-Ghamdi, F.A. Al-Agel, E. Al-Arfaj, F.S. Shokr, S.A. Al-Gahtany, A. Alshahrie, M. Hafez, L.M. Bronstein, G.W. Beall, J. Alloys Compd 640, 122–127 (2015)
Acknowledgements
The authors would like to thank the academy of scientific research and technology (ASRT), in Egypt for Science Up Grant No. 6665.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors have participated in conception, analysis, and interpretation of the data. The authors have participated in revising it critically for important intellectual content and approval of the final version.
Corresponding author
Ethics declarations
Competing interests
All authors declare that they have no conflicts of interest, and the article is the authors' original work, has not received prior publication, and is not under consideration for publication elsewhere.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, A.H., Abbas, Y.M., Aziz, R.A.A. et al. The influence of spin state of the Cr ions on the structural and magnetic behavior of orthorhombic LaFe1−xCrxO3 Perovskites (0.0<x<0.5). J Mater Sci: Mater Electron 34, 830 (2023). https://doi.org/10.1007/s10854-023-10200-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10200-8