Abstract
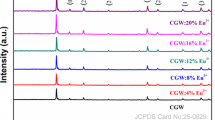
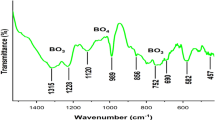
In the present study, the solution combustion reaction method was employed to synthesize the Y8−xSr2(SiO4)6O2:xEu (YSSO:Eu) oxyapatite phosphors where x = 0.10, 0.15, 0.20, 0.25, and 0.30 mol%. The crystal phase study was done using X-Ray Diffraction analysis which confirmed apatite phase formation with hexagonal crystal symmetry and the average crystallite size was found to be 9.35 nm. The FTIR (Fourier Transform Infrared Spectroscopy) spectra were well evident for the bond formations and the presence of functional groups in the prepared sample. Using UV–Visible spectroscopy, band gap values were calculated, and with increasing doping concentrations, the band gap values decreased. The photoluminescence spectral analysis showed peaks at 582 nm, 587 nm, 593 nm, 611 nm, 624 nm, and 675 nm due to transitions of Eu3+ ions from 5D0 (excited state) to 7Fj (j = 0, 1, 2, 3) (ground state), respectively. The Y8−xSr2(SiO4)6O2:0.25Eu oxyapatite phosphor showed the highest intensity peak. The critical distance of energy transfer was calculated to be 4.17 Å implying exchange interaction in the sample. The Judd–Ofelt analysis of synthesized phosphors gave different optical europium transition parameters. The asymmetric environment around the ligand was indicated by the trend, Ω2 > Ω4 followed by J–O parameters. The values of fluorescence branching ratios were found to be more than 0.5 for all doping concentration and the highest calculated lifetime was found to be 9.787 ms for Y8−xSr2(SiO4)6O2:xEu (x = 0.25 mol%) phosphor.















Similar content being viewed by others
Data availability
All relevant data generated or analyzed are included in this article.
References
C.L. Owens, G.R. Nash et al., Apatite enrichment by rare earth elements: A review of the effects of surface properties. Adv. Colloid Interface Sci. 265, 14–28 (2019). https://doi.org/10.1016/j.cis.2019.01.004
P. Ptáček, T. Opravil et al., The field of solid solutions in ternary system of synthetic apatite-type alkaline earth element-yttrium-silicate oxybritholite phases of the composition: AEEδY10δ[SiO4]6O30.5δ, where AEE=Ca, Sr and Ba. Ceram. Int. 42, 6154–6167 (2016). https://doi.org/10.1016/j.ceramint.2016.01.003
S. Yiqiang, D. Zhili et al., Synthesis and crystal structure characterization of silicate apatite Sr2Y8(SiO4)6O2. J. Am. Ceram. Soc. 93, 1176–1182 (2009). https://doi.org/10.1111/j.1551-2916.2009.03563.x
K.B. Steinbru, G.W. Roland et al., Laser properties of Nd3+ and Ho3+ doped crystals with apatite structure. Appl. Opt. 11, 999–1012 (1972)
M. He, H. Shi et al., Immobilization of Pb and Cd in contaminated soil using nano-crystallite hydroxyapatite. Procedia Environ. Sci. 18, 657–665 (2013). https://doi.org/10.1016/j.proenv.2013.04.090
C. Rey, C. Combes et al., Physico-chemical properties of nanocrystalline apatites: Implications for biominerals and biomaterials. Mater. Sci. Eng. C 27, 198–205 (2007). https://doi.org/10.1016/j.msec.2006.05.015
Yu. Ruijin, Na. Xue et al., Photoluminescence characteristics of high thermal stable fluorosilicate apatite Ba2Y3(SiO4)3F: Sm3+ orange-red emitting phosphor. Ceram. Int. 41, 6030–6036 (2015). https://doi.org/10.1016/j.ceramint.2015.01.046
H. Dahiya, M. Dalal et al., Spectroscopic characteristics of Eu3+-activated Ca9Y(PO4)7 nanophosphors in Judd-Ofelt framework. Solid State Sci. 108, 106341 (2020). https://doi.org/10.1016/j.solidstatesciences.2020.106341
S. Singh, S.P. Khatkar et al., Synthesis and optical properties of Gd2(12x) O3: 2xEu3+ nanophosphors via tartaric assisted sol–gel route. J. Sol.-Gel Sci. Technol. 74, 24–31 (2015). https://doi.org/10.1007/s10971-014-3566-3
K. Binnemans, Interpretation of europium (III) spectra. Coord. Chem. Rev. 295, 1–45 (2015). https://doi.org/10.1016/j.ccr.2015.02.015
M. Pokhrel, M. Alcoutlabi et al., Optical and X-ray induced luminescence from Eu3+ doped La2Zr2O7 nanoparticles. J. Alloys Compd. 693, 719–729 (2017). https://doi.org/10.1016/j.jallcom.2016.09.218
Y.Q. Shen, Z.L. Dong et al., Photoluminescence properties and TEM characterization of europium doped silicate oxyapatite Sr2Y8(SiO4)6O2. Microsc. Microanal. Microstruct. 18, 1904 (2012). https://doi.org/10.1017/S1431927612011373
Y. Shen, R. Chen et al., Study of the cation distributions in Eu doped Sr2Y8(SiO4)6O2 by X-ray diffraction and photoluminescent spectra. J. Solid State Chem. 183, 3093–3099 (2010). https://doi.org/10.1016/j.jssc.2010.10.025
Yu. Chunfeng, X. Zhang et al., Determination of Judd-Ofelt parameters for Eu3+-doped alkali borate glasses. Mater. Res. Bull. 120, 110590 (2019). https://doi.org/10.1016/j.materresbull.2019.110590
B.R. Judd, Optical absorption intensities of rare-earth ions. Phys. Rev. Lett. 127, 750–761 (1962). https://doi.org/10.1103/PhysRev.127.750
G.S. Ofelt, Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 37, 511 (1962). https://doi.org/10.1063/1.1701366
S. Qi, Y. Huang et al., Versatile luminescence of Eu2+,3+ activated fluorosilicate apatites M2Y3[SiO4]3F (M = Sr, Ba) suitable for white light emitting diodes. Opt. Mater. Express 4, 396–402 (2014). https://doi.org/10.1364/OME.4.000396
Y. Yang, S. Das et al., Tunable photoluminescence properties and energy transfer in oxyapatite-based Ca2Tb8(SiO4)6:Eu3+ phosphors for UV-LEDs. J. Lumin. 168, 199–206 (2015). https://doi.org/10.1016/j.jlumin.2015.08.009
J. Sokolnicki, E. Zych et al., Synthesis and spectroscopic investigations of Sr2Y8(SiO4)6O2:Eu2+, Eu3+ phosphor for white LEDs. J. Lumin. 158, 65–69 (2015). https://doi.org/10.1016/j.jlumin.2014.09.033
S.V. Motloung, F.B. Dejene et al., Effects of Pb2+ ions concentration on the structure and PL intensity of Pb-doped ZnAl2O4 nanocrystals synthesized using sol–gel process. J. Sol.-Gel Sci. Technol. 70, 422–427 (2014). https://doi.org/10.1007/s10971-014-3302-z
J. Li, Q. Liang et al., Layered structure produced nonconcentration quenching in a novel Eu3+-doped phosphor. ACS Appl. Mater. Interfaces 10, 41479–41486 (2018). https://doi.org/10.1021/acsami.8b13759
J. Mahapatro, S. Agrawal, Effect of Eu3+ ions on electrical and dielectric properties of barium hexaferrites prepared by solution combustion method. Ceram. Int. 47, 20529–20543 (2021). https://doi.org/10.1016/j.ceramint.2021.04.062
M. Rianna, T. Sembiring et al., Microstructure and magnetic properties of BaFe12–2xMgxAlxO19 for microwave absorbing materials. Int. J. Appl. Eng. Res. 12, 6586–6590 (2017)
P. Scherrer, Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen". Nachr. Ges. Wiss. Göttingen 26, 98 (1918)
J.I. Langford, A.J.C. Wilson, Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Cryst. 11, 102 (1978). https://doi.org/10.1107/s0021889878012844
R. Srinivasan, R. Yogamalar et al., Structural and optical studies of yttrium oxide nanoparticles synthesized by co-precipitation method. Mater. Res. Bull. 45, 1165–1170 (2010). https://doi.org/10.1016/j.materresbull.2010.05.020
R. Alimuddin, M. Rafeeq, Synthesis and characterization of strontium oxide nano particle by sol-gel method. Orient. J. Chem. 37, 177–180 (2021). https://doi.org/10.13005/ojc/370124
G. Apsana, P.P. George et al., Biomimetic synthesis and antibacterial properties of strontium oxide nanoparticles using ocimum sanctum leaf extract. Asian J. Pharm. Clin. Res. 11, 384–389 (2018). https://doi.org/10.22159/ajpcr.2018.v11i3.20858
B. Shokri, M. Abbasi Firouzjah et al, FTIR analysis of silicon dioxide thin film deposited by Metal organic-based PECVD
N.J. Shivaramu, B.N. Lakshminarasappa et al., Synthesis characterization and luminescence studies of gamma irradiated nanocrystalline yttrium oxide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 154, 220–231 (2016). https://doi.org/10.1016/j.saa.2015.09.019
N. Sasani, A.H. Khadivi et al., Characterization of rod-like high-purity fluorapatite nanopowders obtained by sol-gel method. J. Ultrafine Grained Nanostruct. Mater. 46, 31–37 (2013)
J. Zhou, R. Lei et al., A new generation of dual-mode optical thermometry based on ZrO2:Eu3+ nanocrystals. Nanophotonics 12, 2347–2358 (2019). https://doi.org/10.1515/nanoph-2019-0359
K. Park, D.H. Kim et al., Crystal structure and photoluminescence properties of garnet−type as−synthesized and aged Li7La3−xZr2O12: xTb phosphors. Ceram. Int. 46, 10194–10202 (2020). https://doi.org/10.1016/j.ceramint.2020.01.011
L.K. Bharat, S.R. Dugasani et al., Preparation of Eu3+ ions activated Ca2La8(SiO4)6O2 oxyapatite nanophosphors through two-step surfactant-free method and their optical and electrical properties. Nanotechnology 28, 375601 (2017). https://doi.org/10.1088/1361-6528/aa7dad
R. Koutavarapu, N. Sita Maha Lakshmi et al., Novel yellow light emission from vanadyl ions-doped calcium-lithium hydroxyapatite nanopowders: structural, optical, and photoluminescence properties. Chem. Pap. 75, 3989–3999 (2021). https://doi.org/10.1007/s11696-021-01632-9
J. Bierwagen, S. Yoon et al., Thermal and concentration dependent energy transfer of Eu2+ in SrAl2O4. Opt. Mater. Express 6, 793–803 (2016). https://doi.org/10.1364/OME.6.000793
S.J. Yoon, J.W. Pi, K. Park, Structural and photoluminescence properties of solution combustionprocessed novel ZrO2 doped with Eu3+ and Al3+. Dyes Pigm. 150, 231–240 (2018). https://doi.org/10.1016/j.dyepig.2017.12.012
K. Park, D.A. Hakeem et al., Improvement of photoluminescence properties of Ce3+ doped CaSrAl2SiO7 phosphors by charge compensation with Li+ and Na+. Ceram. Int. 44, 1929–1934 (2018). https://doi.org/10.1016/j.ceramint.2017.10.135
G. Annadurai, B. Devakumar et al., Novel Eu3+-activated Ba2Y5B5O17 red-emitting phosphors for white LEDs: high color purity, high quantum efficiency and excellent thermal stability. R. Soc. Chem. 8, 23323 (2018). https://doi.org/10.1039/c8ra03059f
A. Ćirić, S. Stojadinović et al., JOES: an application software for Judd-Ofelt analysis from Eu3+ emission spectra. J. Lumin. 205, 351–356 (2019). https://doi.org/10.1016/j.jlumin.2018.09.048
H. Dahiya, M. Dalal et al., Spectroscopic characteristics of Eu3+ activated Ca9Y(PO4)7 nanophosphors in Judd-Ofelt framework. Solid State Sci. 108, 106341 (2020). https://doi.org/10.1016/j.solidstatesciences.2020.106341
P. Phogat, S.P. Khatkar et al., Crystallographic and Judd-Ofelt Parametric investigation into Ca9Bi(VO4)7: Eu3+ nanophosphor for NUV-WLEDs. J. Lumin. 234, 117984 (2021). https://doi.org/10.1016/j.jlumin.2021.117984
A. Ćirić, S. Stojadinović et al., An extension of the Judd-Ofelt theory to the field of lanthanide thermometry. J. Lumin. 216, 116749 (2019). https://doi.org/10.1016/j.jlumin.2019.116749
Q. Chen, B. Miao et al., Enhanced luminescence properties and Judd-Ofelt analysis of novel red emitting Sr2LiScB4O10: Eu3+ phosphors for WLED applications. Opt. Mater. 116, 111093 (2021). https://doi.org/10.1016/j.optmat.2021.111093
J. Hernández-Andrés, R.L. Le et al., calculating correlated color temperatures across the entire gamut of daylight and skylight chromaticities. Appl. Opt. 38, 5703–5709 (1999). https://doi.org/10.1364/AO.38.005703
P. Du, X. Huang, J.S. Yu, Facile synthesis of bifunctional Eu3+ activated NaBiF4 red emitting nanoparticles for simultaneous white light-emitting diodes and field emission displays. Chem. Eng. J. 337, 91–100 (2018). https://doi.org/10.1016/j.cej.2017.12.063
M.M. Rodriguez-Garcia, A. Ciric et al., Narrow-band red phosphors of high color purity based on Eu3+-activated apatite-type Gd9.33(SiO4)6O2. J. Mater. Chem. C 9, 7474 (2021). https://doi.org/10.1039/d1tc01330k
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
RG—Methodology, Software, Formal analysis, Investigation, Data Curation and Writing - Original Draft. SA—Conceptualization, Writing - Review & Editing, Visualization and Supervision.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interests to declare.
Competing interest
The authors declare that they have no known competing financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, R., Agrawal, S. Judd–Ofelt analysis and photoluminescence properties of europium-activated Y8−xSr2(SiO4)6O2 oxyapatite phosphors. J Mater Sci: Mater Electron 33, 17199–17211 (2022). https://doi.org/10.1007/s10854-022-08597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08597-9