Abstract
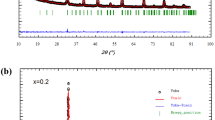
Perovskite manganite La0.5Ca0.5−x□xMnO3 (LCMO) nanomaterials were elaborated using the sucrose modified auto combustion method. Rietveld refinements of the X-ray diffraction patterns of the crystalline structure confirm a single-phase orthorhombic state with Pbnm space group (No. 62). The Ca-vacancies were voluntarily created in the LCMO structure in order to study their influence on the magnetic behaviour in the system. The magnetic susceptibility was found to be highly enhanced in the sample with Ca-vacancies. Paramagnetic-to-ferromagnetic phase transition was evidenced in both samples around 254 K. This transition is, characterized by a drastic jump of the susceptibility in the sample with Ca-vacancies. The maximum of entropy change, observed for both compounds at magnetic field of 6 T was 2.30 J kg−1 K−1 and 2.70 J kg−1 K−1 for the parent compound and the lacunar one respectively. The magnetocaloric adiabatic temperature change value calculated by indirect method was 5.6 K and 5.2 K for the non-lacunar and Ca-vacancy compound, respectively. The Ca-lacunar La0.5Ca0.5−x□xMnO3 (x = 0.05) reported in this work demonstrated overall enhancement of the magnetocaloric effect over the LCMO. The technique used to elaborate LCMO materials was beneficial to enhance the magnetocaloric effect and magnetic behaviour. Therefore, we conclude that this less costly environmentally friendly system can be considered as more advantageous candidate for magnetic refrigeration applications then the commonly Gd-based compounds.











Similar content being viewed by others
References
V. Franco, J.S. Blázquez, J.J. Ipus, J.Y. Law, L.M. Moreno-Ramírez, A. Conde, Magnetocaloric effect: from materials research to refrigeration devices. Prog. Mater Sci. 93, 112–232 (2018). https://doi.org/10.1016/j.pmatsci.2017.10.005
M.A. Benedict, S.A. Sherif, M. Schroeder, D.G. Beers, The impact of magnetocaloric properties on refrigeration performance and machine design. Int. J. Refrig 74, 576–583 (2017). https://doi.org/10.1016/j.ijrefrig.2016.12.004
D.J. Silva, B.D. Bordalo, J. Puga, A.M. Pereira, J. Ventura, J.C.R.E. Oliveira, J.P. Araújo, Optimization of the physical properties of magnetocaloric materials for solid state magnetic refrigeration. Appl. Therm. Eng. 99, 514–517 (2016). https://doi.org/10.1016/j.applthermaleng.2016.01.026
J. Rodríguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192, 55–69 (1993). https://doi.org/10.1016/0921-4526(93)90108-I
J. Yang, W.H. Song, Y.Q. Ma, R.L. Zhang, Y.P. Sun, Determination of oxygen stoichiometry in the mixed-valent manganites. J. Magn. Magn. Mater. 285, 417–421 (2005). https://doi.org/10.1016/j.jmmm.2004.08.012
J.M. González-Calbet, E. Herrero, N. Rangavittal, J.M. Alonso, J.L. Martínez, M. Vallet-Regí, Ordering of Oxygen Vacancies and Magnetic Properties in La0.5Ca0.5MnO3 − δ (0 ≤ δ≤0.5). J. Solid State Chem. 148, 158–168 (1999). https://doi.org/10.1006/jssc.1999.8441
I. Walha, H. Ehrenberg, H. Fuess, A. Cheikhrouhou, Structure and magnetic properties of lanthanum and calcium-deficient La0.5Ca0.5MnO3 manganites. J. Alloys Compd. 433, 63–67 (2007). https://doi.org/10.1016/j.jallcom.2006.06.095
M. Ghahremani, A. Aslani, M. Hosseinnia, L.H. Bennett, E. Della Torre, Direct and indirect measurement of the magnetocaloric effect in bulk and nanostructured Ni-Mn-In Heusler alloy. AIP Adv. 8, 056426 (2018)
A.O. Pecharsky, K.A. Gschneidner, V.K. Pecharsky, The giant magnetocaloric effect of optimally prepared Gd5Si2Ge2. J. Appl. Phys. 93, 4722–4728 (2003). https://doi.org/10.1063/1.1558210
V.K. Pecharsky, K.A. Gschneidner, A.O. Pecharsky, A.M. Tishin, Thermodynamics of the magnetocaloric effect. Phys. Rev. B. 64, 144406 (2001). https://doi.org/10.1103/PhysRevB.64.144406
V.K. Pecharsky, K.A. Gschneidner Jr., Magnetocaloric effect and magnetic refrigeration. J. Magn. Magn. Mater. 200, 44–56 (1999). https://doi.org/10.1016/S0304-8853(99)00397-2
K. Das, N. Banu, I. Das, B.N. Dev, Significantly large magnetocaloric effect in polycrystalline La0.83Sr0.17MnO3 near room temperature. Physica B 545, 438–441 (2018). https://doi.org/10.1016/j.physb.2018.06.029
S.C. Das, K. Mandal, P. Dutta, S. Pramanick, S. Chatterjee, Observation of room temperature magnetocaloric effect in Mn0.9Ni1.1Ge alloy. J. Magn. Magn. Mater. 465, 44–47 (2018). https://doi.org/10.1016/j.jmmm.2018.05.077
M. Baldini, T. Muramatsu, M. Sherafati, H. Mao, L. Malavasi, P. Postorino, S. Satpathy, V.V. Struzhkin, Origin of colossal magnetoresistance in LaMnO3 manganite. Proc. Natl. Acad. Sci. USA 112, 10869–10872 (2015). https://doi.org/10.1073/pnas.1424866112
P. Rivero, V. Meunier, W. Shelton, Electronic, structural, and magnetic properties of LaMnO3 phase transition at high temperature. Phys. Rev. B. (2016). https://doi.org/10.1103/physrevb.93.024111
J. Makni-Chakroun, W. Cheikhrouhou-Koubaa, M. Koubaa, A. Cheikhrouhou, Impact of a small amount of vacancy in both lanthanum and calcium on the physical properties of nanocrystalline La0.7Ca0.3MnO3 manganite. J. Alloys Compd. 650, 421–429 (2015). https://doi.org/10.1016/j.jallcom.2015.07.052
A. Ezaami, I. Sfifir, W. Cheikhrouhou-Koubaa, M. Koubaa, A. Cheikhrouhou, Critical properties in La0.7Ca0.2Sr0.1MnO3 manganite: a comparison between sol-gel and solid state process. J. Alloys Compd. 693, 658–666 (2017). https://doi.org/10.1016/j.jallcom.2016.09.223
A. Jerbi, A. Krichene, R. Thaljaoui, W. Boujelben, Structural, magnetic, and electrical study of polycrystalline Pr0.55Sr0.45 − x Na x MnO3 (x = 0.05 and 0.1). J. Supercond. Novel Magn. 29, 123–132 (2016). https://doi.org/10.1007/s10948-015-3217-0
A. Gómez, E. Chavarriaga, I. Supelano, C.A. Parra, O. Morán, Tuning the magnetocaloric properties of La0.7Ca0.3MnO3 manganites through Ni-doping. Phys. Lett. A. 382, 911–919 (2018). https://doi.org/10.1016/j.physleta.2018.01.030
Y. Tomioka, T. Okuda, Y. Okimoto, A. Asamitsu, H. Kuwahara, Y. Tokura, Charge/orbital ordering in perovskite manganites. J. Alloy. Compd. 326, 27–35 (2001). https://doi.org/10.1016/S0925-8388(01)01222-1
Z.-J. Yao, W.-Q. Chen, J.-H. Gao, H.-M. Jiang, F.-C. Zhang, Theory for charge and orbital density-wave states in manganite La0.5Sr1.5MnO4. Phys. Rev. B. (2013). https://doi.org/10.1103/physrevb.87.155103
L. Yu-Kuai, Y. Yue-Wei, L. Xiao-Guang, Colossal magnetoresistance in manganites and related prototype devices. Chin. Phys. B. 22, 087502 (2013). https://doi.org/10.1088/1674-1056/22/8/087502
H.A. Jahn, E. Teller, Stability of polyatomic molecules in degenerate electronic states: I—orbital degeneracy. Proc. R. Soc. Lond. A. 161, 220–235 (1937). https://doi.org/10.1098/rspa.1937.0142
V.M. Andrade, R.J.C. Vivas, S.S. Pedro, J.C.G. Tedesco, A.L. Rossi, A.A. Coelho, D.L. Rocco, M.S. Reis, Magnetic and magnetocaloric properties of La0.6Ca0.4MnO3 tunable by particle size and dimensionality. Acta Mater. 102, 49–55 (2016). https://doi.org/10.1016/j.actamat.2015.08.080
Y. Regaieg, M. Koubaa, W.C. Koubaa, A. Cheikhrouhou, L. Sicard, S. Ammar-Merah, F. Herbst, Structure and magnetocaloric properties of La0.8Ag0.2 − xKxMnO3 perovskite manganites. Mater. Chem. Phys. 132, 839–845 (2012). https://doi.org/10.1016/j.matchemphys.2011.12.021
M. Pękała, V. Drozd, Magnetocaloric effect in nano- and polycrystalline. J. Non-Crystalline Solids. 354, 5308–5314 (2008). https://doi.org/10.1016/j.jnoncrysol.2008.06.112
A. Krichene, W. Boujelben, A. Cheikhrouhou, Structural, magnetic and magnetocaloric properties in La0.5 − xRexCa0.5MnO3 manganites (x = 0; 0.1 and Re = Gd, Eu and Dy). J. Alloys Compd. 550, 75–82 (2013). https://doi.org/10.1016/j.jallcom.2012.09.036
H. Gencer, N.E. Cengiz, V.S. Kolat, T. Izgi, S. Atalay, Production of LaCaMnO3 composite by ball milling. Acta Phys. Pol. A 125, 214–216 (2014). https://doi.org/10.12693/APhysPolA.125.214
J.Q. Li, W.A. Sun, W.Q. Ao, J.N. Tang, Hydrothermal synthesis and Magnetocaloric effect of La0.5Ca0.3Sr0.2MnO3. J. Magn. Magn. Mater. 302, 463–466 (2006). https://doi.org/10.1016/j.jmmm.2005.10.007
A. Arabi, M. Fazli, M.H. Ehsani, Tuning the morphology and photocatalytic activity of La0.7Ca0.3MnO3 nanorods via different mineralizer-assisted hydrothermal syntheses. Mater. Res. Bull. 90, 205–211 (2017). https://doi.org/10.1016/j.materresbull.2017.02.043
A. Mrakovic, J. Blanusă, D. Primc, M. Perovic, Z. Jagličic, V. Kusigerski, V. Spasojevic, Modified self-propagating high-temperature synthesis of nanosized La0.7Ca0.3MnO3. Ceram. Int. (2012). https://doi.org/10.1016/j.ceramint.2012.10.216
D. Berger, C. Matei, F. Papa, D. Macovei, V. Fruth, J.P. Deloume, Pure and doped lanthanum manganites obtained by combustion method. J. Eur. Ceram. Soc. 27, 4395–4398 (2007). https://doi.org/10.1016/j.jeurceramsoc.2007.02.164
K.P. Shinde, N.D. Thorat, S.S. Pawar, S.H. Pawar, Combustion synthesis and characterization of perovskite La0.9Sr0.1MnO3. Mater. Chem. Phys. 134, 881–885 (2012). https://doi.org/10.1016/j.matchemphys.2012.03.085
S. Ben Moumen, Y. Gagou, S. Belkhadir, D. Mezzane, M. Amjoud, B. Rozic, L. Hajji, Z. Kutnjak, Z. Jaglicic, M. Jagodic, M. El Marssi, Y. Kopelevich, I.A. Luk’yanchuk, Structural, dielectric and magnetic properties of multiferroic (1-x) La0.5Ca0.5MnO3-(x) BaTi0.8Sn0.2O3 laminated composites. IEEE Trans. Ultrason. Ferroelectr. Freq. Control (2019). https://doi.org/10.1109/tuffc.2019.2935459
M. Smari, I. Walha, E. Dhahri, E.K. Hlil, Structural, magnetic and magnetocaloric properties of Ag-doped La0.5Ca0.5 − Ag MnO3 compounds with 0 ≤x≤ 0.4. J. Alloys Compd. 579, 564–571 (2013). https://doi.org/10.1016/j.jallcom.2013.07.104
A. Elghoul, A. Krichene, N. Chniba Boudjada, W. Boujelben, Rare earth effect on structural, magnetic and magnetocaloric properties of La0.75Ln0.05Sr0.2MnO3 manganites. Ceram. Int. 44, 12723–12730 (2018). https://doi.org/10.1016/j.ceramint.2018.04.075
K. Momma, F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011). https://doi.org/10.1107/S0021889811038970
S. Chaffai, W. Boujelben, M. Ellouze, A. Cheikh-rouhou, J.C. Joubert, A comparative study of the physical properties of Pr0.5 − x□xSr0.5MnO3 and Pr0.5Sr0.5 − x□xMnO3 manganites. Physica B 321, 74–78 (2002). https://doi.org/10.1016/s0921-4526(02)00825-6
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 32, 751–767 (1976). https://doi.org/10.1107/S0567739476001551
M. Khlifi, M. Bejar, O. EL Sadek, E. Dhahri, M.A. Ahmed, E.K. Hlil, Structural, magnetic and magnetocaloric properties of the lanthanum deficient in La0.8Ca0.2 − x□xMnO3 (x = 0–0.20) manganites oxides. J. Alloys Compd. 509, 7410–7415 (2011). https://doi.org/10.1016/j.jallcom.2011.04.049
W. Boujelben, A. Cheikh-Rouhou, J. Pierre, J.C. Joubert, Ferromagnetism in the lacunar (Pr, Sr)MnO3 perovskite manganites. Physica B 321, 37–44 (2002). https://doi.org/10.1016/S0921-4526(02)00818-9
C. Kittel, Introduction to solid state physics, 6th edn. (Wiley, New York, 1986)
N. Abdelmoula, A. Cheikh-Rouhou, L. Reversat, Structural, magnetic and magnetoresistive properties of La0.7Sr0.3-xNaxMnO3 manganites. J. Phys. Condens. Matter. 13, 449 (2001). https://doi.org/10.1088/0953-8984/13/3/307
Y.A. Izyumov, Y.N. Skryabin, Double exchange model and the unique properties of the manganites. Phys. Usp. 44, 109 (2001). https://doi.org/10.1070/PU2001v044n02ABEH000840
C. Zener, Interaction between the $d$-shells in the transition metals. II. Ferromagnetic compounds of manganese with perovskite structure. Phys. Rev. 82, 403–405 (1951). https://doi.org/10.1103/physrev.82.403
W. Tang, W. Lu, X. Luo, B. Wang, X. Zhu, W. Song, Z. Yang, Y. Sun, Particle size effects on La0.7Ca0.3MnO3: size-induced changes of magnetic phase transition order and magnetocaloric study. J. Magn. Magn. Mater. 322, 2360–2368 (2010). https://doi.org/10.1016/j.jmmm.2010.02.038
P. Levy, F. Parisi, G. Polla, D. Vega, G. Leyva, H. Lanza, R.S. Freitas, L. Ghivelder, Controlled phase separation in La0.5Ca0.5MnO3. Phys. Rev. B. 62, 6437–6441 (2000). https://doi.org/10.1103/physrevb.62.6437
I. Walha, H. Ehrenberg, H. Fuess, A. Cheikhrouhou, Structural and magnetic properties of La0.6 − x□xCa0.4MnO3 (0 ≤ x ≤ 0.2) perovskite manganite. J. Alloys Compd. 485, 64–68 (2009). https://doi.org/10.1016/j.jallcom.2009.06.121
M. Mansouri, H. Omrani, W. Cheikhrouhou-Koubaa, M. Koubaa, A. Madouri, A. Cheikhrouhou, Effect of vanadium doping on structural, magnetic and magnetocaloric properties of La0.5Ca0.5MnO3. J. Magn. Magn. Mater. 401, 593–599 (2016). https://doi.org/10.1016/j.jmmm.2015.10.066
M. Khlifi, E. Dhahri, E.K. Hlil, Magnetic, magnetocaloric, magnetotransport and magnetoresistance properties of calcium deficient manganites La0.8Ca0.2 − x□xMnO3 post-annealed at 800 °C. J. Alloys Compd. 587, 771–777 (2014). https://doi.org/10.1016/j.jallcom.2013.11.012
Acknowledgements
The authors gratefully acknowledge the financial support of the European H2020-MSCA-RISE-2017-ENGIMA action and the CNRST Priority Program PPR 15/2015.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ben Moumen, S., Gagou, Y., Chettab, M. et al. Synthesis of La0.5Ca0.5−x□xMnO3 nanocrystalline manganites by sucrose assisted auto combustion route and study of their structural, magnetic and magnetocaloric properties. J Mater Sci: Mater Electron 30, 20459–20470 (2019). https://doi.org/10.1007/s10854-019-02392-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02392-9