Abstract
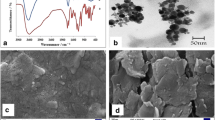
A simple and sensitive electrochemical determination of l-vanillin (VA), a powerful anti-microbial agent and a commonly employed food preservative was investigated using copper hexacyanoferrate (CuHCF) film modified on 2-mercaptoethylamine functionalized colloidal gold nanoparticles (colloid-Au) graphite-wax composite electrode. As achieved surface modification was characterized using confocal Raman spectroscopy studies which gave rise to peaks at 351, 493, 807 and 2048 cm−1 that corresponds to the characteristics vibrational modes of ν(Fe–C ≡N–Cu), ν(Cu–N), ν(Fe–C) and ν(–C≡N) for the CuHCF film. Further, the elemental analysis investigated using X-ray photoelectron spectroscopy confirms the presence of 2p peaks of Cu (II), Fe(II) and 1 s edge peaks of N for the CuHCF film modified electrode. The modified electrode exhibited a high sensitivity of 0.1817 μA μM−1 at a lower detection potential of 0.68 V towards the VA determination and the limit of detection was observed to be 2.53 × 10−7 M (S/N = 3). The VA content in commercial roasted coffee beans was also examined with the proposed sensor and the results were found to be satisfactory.









Similar content being viewed by others
References
D.J. Fitzgeral, M. Stratford, A. Narbad, Int. J. Food Microbiol. 86, 113–122 (2003)
H. Ketli, L.S. Yazan, N. Ismail, M. Ismail, Food Chem. Toxicol. 49, 25–30 (2011)
H.G. Sammons, R.T. Williams, J. Biochem. 35, 1175–1189 (1941)
N.J. Walton, M.J. Mayer, A. Narbad, Phytochemistry 63, 505–515 (2003)
J. Adedeji, T.G. Hartman, C. Ho, Perfum. Flavor. 18, 25 (1993)
G. Lamprecht, F. Pichlmayer, E.R. Schmid, J. Agric. Food Chem. 42, 1722–1727 (1994)
A. Herrmann, M. Stockli, J. Chromgr. 246, 313–316 (1982)
A. Scharrer, A. Mosandi, Dtsch. Lebensm. Rundsch. 97, 449–456 (2001)
M. Ohashi, H. Omae, M. Hashida, Y. Sowa, S. Imai, J. Chromgr. A 1138, 262–267 (2007)
W. Tao, D. Pan, Y. Liu, L. Nie, S. Yao, J. Electroanal. Chem. 572, 109–117 (2004)
F. Wang, J. Wang, H. Chen, S. Dong, J. Electroanal. Chem. 600, 265–274 (2007)
P. Deng, Z. Xu, R. Zeng, C. Ding, Food Chem. 180, 156–163 (2015)
V. Veeramani, R. Madhu, S.M. Chem, P. Veerakumar, J.J. Syu, S.B. Liu, New J. Chem. 39, 9109–9115 (2015)
G. Ziyatdinova, E. Kozlova, E. Ziganshina, H. Budnikov, Monatshefte fur Chem. 147, 191–200 (2016)
L. Shang, F. Zhao, B. Zeng, Food Chem. 151, 53–57 (2014)
X. Wang, C. Luo, L. Li, H. Duan, RSC Adv. 5, 92932–92939 (2015)
F. Bettazzi, I. Palchetti, S. Sisalli, M. Mascini, Anal. Chim. Acta 555, 134–138 (2006)
L. Agui, J.E. Lopez-Guzman, A. Gonzalez-Cortes, P. Yanez-Sedeno, J.M. Pingarron, Anal. Chim. Acta 385, 241–248 (1999)
J.L. Hardcastle, C.J. Paterson, R.G. Compton, Electroanalysis 13, 899–905 (2001)
E.G. Cookeas, C.E. Efstathiou, Analyst 117, 1329–1334 (1992)
M. Luque, E. Luque-Perez, A. Rios, M. Valcarce, Anal. Chim. Acta 410, 127–134 (2000)
P. Prabhu, R.S. Babu, S.S. Narayanan, Coll. Surf. B 87, 103–108 (2011)
K.R. Brown, D.G. Walter, M.J. Natan, Chem. Mater. 12, 306–313 (2000)
J. Wright, M.M. Barsan, I.S. Butler, J. Fitzpatrick, D.F.R. Gilson, M.O. Adebajo, R.L. Frost, J. Raman Spectrosc. 42, 1562–1566 (2011)
A.P. Grosvenor, B.A. Kobe, M.C. Biesinger, N.S. McIntyre, Surf. Interface Anal. 36, 1564–1574 (2004)
S. Tricard, F. Charra, T. Mallah, Dalton Trans. 42, 15835–15845 (2013)
P. Semmelroch, G. Laskawy, I. Blank, W. Groscht, Flavour. Fragr. J. 10, 1–7 (1995)
Acknowledgements
PP and SSN acknowledges the financial assistance from University Grants Commission (UGC), New Delhi, India by the way of Project Fellow and Basic Scientific Research (BSR) fellowships and Department of Science & Technology for DST-PURSE program in support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prabhu, P., Babu, R.S. & Narayanan, S.S. Electrochemical determination of l-vanillin using copper hexacyanoferrate film modified gold nanoparticle graphite-wax composite electrode. J Mater Sci: Mater Electron 30, 9955–9963 (2019). https://doi.org/10.1007/s10854-019-01335-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01335-8