Abstract
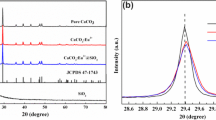
In this study, the fusiform Co-doped CeO2 particles were prepared by a hydrothermal method and characterized by X-ray diffraction (XRD), thermo-gravimetric differential thermal analysis (TG–DTA), scanning electron microscope (SEM), transmission electron microscope (TEM), energy dispersive X-ray spectrometer (EDX), X-ray photo-electron spectroscopy (XPS), Raman, photoluminescence (PL) and UV–Vis spectroscopy. The cubic fluorite structure of CeO2 were supported by XRD. Initially thermal gravimetric and differential thermal analysis were used to analyse the reaction mechanism and chemical process, which indicated a crystal temperature of the as-synthesized CeO2 particles at 600 °C. The reaction concentration of the solution were systematically researched. It is found that the reaction concentration is one key parameter for controlling the final micrographs. The optical properties were characterized by Raman, PL and UV–Vis spectroscopy. It has been demonstrated that oxygen vacancies and Ce3+ exist at the particle surfaces and boundaries. The appearance of luminescence peaks and emission peaks can be ascribed to the related oxygen defects. The appearance of Ce3+ and oxygen defects existing in fusiform Co-doped CeO2 products can cause the formation of several local band gap states, which is perhaps responsible for the red shift of band gap.










Similar content being viewed by others
References
B. Sun, C.M. Li, Phys. Chem. Chem. Phys. 17, 6718 (2015)
A. Sobhani-Nasab, M. Maddahfar, S.M. Hosseinpour-Mashkani, J. Mol. Liq. 216, 1 (2016)
J.G. Lv, J.H. Xu, M. Zhao, Ceram. Int. 41, 13983–13987 (2015)
Z. Fan, F. Meng, M. Zhang, Z. Wu, Z. Sun, A. Li, Appl. Surf. Sci. 360, 298 (2016)
H.F. Zhang, X. He, Z.Y. Zhang, P. Zhang, Y.Y. Li, Y.H. Ma, Y.S. Kuang, Y.L. Zhao, Z.F. Chai, Environ. Sci. Technol. 45, 3730 (2011)
M. Zhang, Y.Y. Xu, Z.Z. Gong, J. Alloys Compd. 649, 190–195 (2015)
L. Weijia Han, X. Ren, Qi, Appl. Surf. Sci. 299, 12–18 (2014)
E. Tang, G. Cheng, X. Ma, Powder Technol. 161, 209 (2006)
F. Esch, S. Fabris, L. Zhou, T. Montini, C. Africh, P.F.G. Comelli, R. Rosei, Science 309, 752 (2005)
L.N. Wang, F.M. Meng, K.K. Li, F. Lu, Appl. Surf. Sci. 286, 269 (2013)
H.R. Tan, J.P.Y. Tan, C. Boothroyd, T.W. Hansen, Y.L. Foo, M. Lin, J. Phys. Chem. C 116, 242 (2012)
H. Imagawa, A. Suda, K. Yamamura, S.H. Sun, J. Phys. Chem. C 115, 1740 (2011)
C.T. Campbell, C.H.F. Peden, Science 309, 713 (2005)
P. Bera, A. Gayen, M.S. Hegde, N.P. Lalla, L. Spadaro, F. Frusteri, F. Arena, J. Phys. Chem. B 107, 6122 (2003)
Z.L. Wang, Z.W. Quan, J. Lin, Inorg. Chem. 46, 5237 (2007)
X.D. Zhou, W. Huebner, Appl. Phys. Lett. 79, 3512–3514 (2001)
R.V. Barde, Spectrochim. Acta A 153, 160 (2016)
X.M. Qu, L.X. You, X.C. Tian, B.W. Zhang, G.D. Mahadevan, Y.X. Jiang, S.G. Sun, Electrochim. Acta 182, 1078 (2015)
Y. Liu, H. Huang, L.L. Wang, D.P. Cai, B. Liu, D.D. Wang, Q.H. Li, T.H. Wang, Sens. Actuators B 223, 730 (2016)
S.S. Gu, Y.N. Chen, X.H. Yuan, H. Wang, X.H. Chen, Y. Liu, Q. Jiang, Z.B. Wu, G.M. Zeng, RSC Adv. 5, 79556 (2015)
Q.H. Bo, F.M. Meng, L.N. Wang, Mater. Lett. 133, 216 (2014)
R.B. Yu, L. Yan, P. Zheng, J. Chen, X.R. Xing, J. Phys. Chem. C 112, 19896 (2008)
X.W. Lu, X.Z. Li, F. Chen, C.Y. Ni, Z.G. Chen, J. Alloys Compd. 476, 958 (2009)
C. Paun, O.V. Safonova, J. Szlachetko, P.M. Abdala, M. Nachtegaal, J. Sa, E. Kleymenov, A. Cervellino, F. Krumeich, J.A. van Bokhoven, J. Phys. Chem. C 116, 7312 (2012)
M. Jobbagy, F. Marino, B. Schonbrod, G. Baronetti, M. Laborde, Chem. Mater. 18, 1945–1950 (2006)
H. Xiao, Z. Ai, L. Zhang, J. Phys. Chem. C 113, 16625 (2009)
L. Xu, H. Song, B. Dong, Y. Wang, J. Chen, X. Bai, Inorg. Chem. 49, 10590 (2010)
D. Arumugam, M. Thangapandian, A. Jayaram, G.S. Okram, N.P. Lalla, M.F.B. Amirtham, J. Phys. Chem. C 120, 26544 (2016)
F.M. Meng, H.J. Li, J.F. Gong, Z.H. Fan, J. Mater. Sci. Mater. Electron. 27, 8433 (2016)
S. Colis, A. Bouaine, G. Schmerber, C. Ulhaq-Bouillet, A. Dinia, S. Choua, P. Turek, Phys. Chem. Chem. Phys. 14, 7256 (2012)
J.R. McBride, K.C. Hass, B.D. Poindexter, W.H. Weber, J. Appl. Phys. 76, 2435 (1994)
N.J. Lawrence, J.R. Brewer, L. Wang, T.S. Wu, J. Wells-Kingsbury, M.M. Ihrig, G.H. Wang, Y.L. Soo, W.N. Mei, C.L. Cheung, Nano Lett. 11, 2666 (2011)
Z.P. Li, F.C. Han, C. Li, X.L. Jiao, D.R. Chen, RSC Adv. 42, 60975 (2016)
T. Hattori, K. Kobayashi, M. Ozawa, Jpn. J. Appl. Phys. 56, 01 (2017)
B. Choudhury, A. Choudhury, Mater. Chem. Phys. 131, 666 (2012)
K.S. Hemalatha, K. Rukmani, RSC Adv. 6, 74354 (2016)
I.O. Mazali, B.C. Viana, O.L. Alves, J.M. Filho, A.G. Souza Filho, J. Phys. Chem. Solids 68, 622 (2007)
S.B. Khan, M. Faisal, M.M. Rahman, A. Jamal, Sci. Total Environ. 409, 2987 (2011)
C.W. Sun, H. Li, H.R. Zhang, Z.X. Wang, L.Q. Chen, Nanotechnology 16, 1454 (2005)
X.D. Li, J.G. Li, D. Huo, Z.M. Xiu, X.D. Sun, J. Phys. Chem. C 113, 1806 (2009)
Acknowledgements
This work was supported by the Anhui Provincial Natural Science Foundation of China (1508085SME219).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, P., Meng, F., Gao, C. et al. Structural, morphological and optical characteristics of fusiform Co-doped CeO2 via a facile hydrothermal method. J Mater Sci: Mater Electron 29, 11482–11488 (2018). https://doi.org/10.1007/s10854-018-9243-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9243-5