Abstract
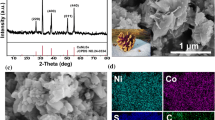
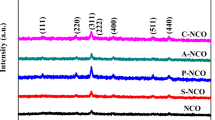
Morphology control of electrode material is critical for high performance supercapacitors. The synthesis of shape-controlled materials is helpful to design advanced electrode materials for supercapacitors. Herein, we prepared two types of NiCo2S4 with nano-bud and nano-mesh morphologies under hydrothermal conditions by facile controlling the reaction time. These materials display morphology-dependent electrochemical activity. Electrochemical measurements showed that the nano-mesh-like NiCo2S4 demonstrate the superior pseudocapacitive performance with a high specific capacitance (3.00 F cm−2 or 1250 F g−1 at 2 mA cm−2) and a favorable rate capability (77.8% from 2 to 25 mA cm−2). Moreover, the specific capacitance remains 80% of its initial value after 5000 cycles even at high current density of 20 mA cm−2. The mesh-like NiCo2S4 also displays a low overpotentials of 299 mV at the current density of 30 mA cm−2, showing better performance in electrochemical oxygen evolution reaction.









Similar content being viewed by others
References
C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang, J. Zhang, Chem. Soc. Rev. 44, 7484–7539 (2015)
H. Chen, J. Jiang, L. Zhang, H. Wan, T. Qi, D. Xia, Nanoscale 5, 8879–8883 (2013)
M. Sun, J. Tie, G. Cheng, T. Lin, S. Peng, F. Deng, F. Ye, L. Yu, J. Mater. Chem. A 3, 1730–1736 (2015)
J. Yin, H. Zhang, J. Luo, M. Yao, W. Hu, J. Mater. Sci.: Mater. Electron. 28, 2093–2099 (2016)
Y.M. Zhang, Y.W. Sui, J.Q. Qi, P.H. Hou, F.X. Wei, Y.Z. He, Q.K. Meng, Z. Sun, J. Mater. Sci.: Mater. Electron. 28, 5686–5695 (2016)
R. Ding, H. Gao, M. Zhang, J. Zhang, X. Zhang, RSC Adv. 5, 48631–48637 (2015)
S. Chen, H.C. Chen, M.Q. Fan, C. Li, K.Y. Shu, J. Sol-Gel Sci. Techol. 80, 119–125 (2016)
H. Chen, J. Jiang, Y. Zhao, L. Zhang, D. Guo, D. Xia, J. Mater. Chem. A 3, 428–437 (2015)
Y. Zhu, X. Ji, Z. Wu, Y. Liu, Electrochim. Acta 186, 562–571 (2015)
F. Deng, L. Yu, M. Sun, T. Lin, G. Cheng, B. Lan, F. Ye, Electrochim. Acta 133, 382–390 (2014)
S. Peng, L. Yu, M. Sun, G. Cheng, T. Lin, Y. Mo, Z. Li, J. Power Sources 296, 237–244 (2015)
Z. Ai, Z. Hu, Y. Liu, M. Yao, ChemPlusChem 81, 322–328 (2016)
J. Xiao, L. Wan, S. Yang, F. Xiao, S. Wang, Nano Lett. 14, 831–838 (2014)
Z. Lv, Q. Zhong, Z. Zhao, Y. Bu, J. Mater. Sci.: Mater. Electron. (2017). doi:10.1007/s10854-017-7750-4
L. Shen, J. Wang, G. Xu, H. Li, H. Dou, X. Zhang, Adv. Energy Mater. 5, 1400977 (2015)
W. Chen, C. Xia, H.N. Alshareef, ACS Nano 8, 9531–9541 (2014)
J.C. Liang, P. Sun, G.Y. Chen, W.X. Zhang, K.Y. Zhou, W.Z. Zhang, J.A. Liu, Z.P. Zhang, M. Zhou, W. Hou, F. Niu, J. Mater. Sci.: Mater. Electron. 28, 14646–14654 (2017)
L. Shen, L. Yu, H.B. Wu, X.Y. Yu, X. Zhang, X.W. Lou, Nat. Commun. 6, 6694 (2015)
L. Yu, L. Zhang, H.B. Wu, X.W. Lou, Angew. Chem. Int. Ed. 53, 3711–3714 (2014)
L.G. Beka, X. Li, W.H. Liu, J. Mater. Sci.: Mater. Electron. 27, 10894–10904 (2016)
Y. Zhang, M. Ma, J. Yang, C. Sun, H. Su, W. Huang, X. Dong, Nanoscale 6, 9824–9830 (2014)
L. Hou, H. Hua, R. Bao, Z. Chen, C. Yang, S. Zhu, G. Pang, L. Tong, C. Yuan, X. Zhang, ChemPlusChem 81, 557–563 (2016)
X.H. Xiong, G. Waller, D. Ding, D.C. Chen, B. Rainwater, B.T. Zhao, Z.X. Wang, M.L. Liu, Nano Energy 16, 71–80 (2015)
T.Y. Wei, C.H. Chen, H.C. Chien, S.Y. Lu, C.C. Hu, Adv. Mater. 22, 347–351 (2010)
Y. Xue, Z. Zuo, Y. Li, H. Liu, Small 13, 1700936 (2017)
Z. Wang, J. Li, X. Tian, X. Wang, Y. Yu, K.A. Owusu, L. He, L. Mai, ACS Appl. Mater. Interfaces 8, 19386–19392 (2016)
J. Jiang, C. Yan, X. Zhao, H. Luo, Z. Xue, T. Mu, Green Chem. 19, 3023–3031 (2017)
Acknowledgements
This work was financially supported by the Scientific Program of Guangdong Province (2014A010106030, 2016A010104017, 2016B020241003, 2016B090930004), the Foundation of Higher Education of Guangdong Province (2015KTSCX027).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tie, J., Peng, S., Diao, G. et al. Shape-controlled synthesis of nickel–cobalt–sulfide with enhanced electrochemical activity. J Mater Sci: Mater Electron 29, 2251–2258 (2018). https://doi.org/10.1007/s10854-017-8140-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8140-7