Abstract
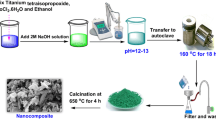
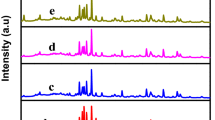
MnCo2O4 nano-composite photocatalyst was synthesized via co-precipitation technique. Composite formation of cobalt oxide with manganese was intended to create tail states within the band gap of cobalt oxide for better charge separation of the photo-generated electron–hole pairs in nano-composite photocatalyst. The MnCo2O4 nano-composite can effectively mineralize organic contaminants. MnCo2O4 nano-composite photocatalyst was characterized by X-ray diffraction, field emission scanning electron microscopy (FESEM), transmission electron microscopy, energy dispersive X-ray spectroscopy, fourier transform infrared spectroscopy (FTIR) and UV–Vis diffuse reflectance spectra (UV–Vis DRS). X-ray diffraction analysis showed cubic manganese cobalt oxide MnCo2O4. FESEM results showed spherical nanoparticles with cauliflower nanostructure. The mean size of particles was in the ranges of 30–60 nm. The band gap for MnCo2O4 nano-composite photocatalyst was 1.95 and 2.49 eV, based on the results of diffuse reflectance spectra. FTIR results showed two type of fundamental absorption bands at 620 and 505 cm−1 due to stretching vibrations of Mn–O and Co–O, respectively. The photo-catalytic activitie of nano-composite photocatalysts were investigated using a naphthol blue black dye as textile dye contaminant and the results showed an outstanding performance yielded 92 % total organic carbon removals attributed to charge separation of the photo-generated electron–hole pairs in nano-composite.











Similar content being viewed by others
References
Y.M. Hunge, V.S. Mohite, S.S. Kumbhar, K.Y. Rajpure, A.V. Moholkar, H. Bhosale, J. Mater. Sci.: Mater. Electron. 26, 8404 (2015)
D. Ghanbari, S. Sharifi, A. Naraghi, G. Nabiyouni, J. Mater. Sci.: Mater. Electron. 27, 5315 (2016)
S. Moshtaghi, S. Gholamrezaei, M. Salavati Niasari, P. Mehdizadeh, J. Mater. Sci: Mater. Electron 27, 414 (2016)
F. Beshkar, O. Amiri, M. Salavati-Niasari, F. Beshkar, J. Mater. Sci.: Mater. Electron. 26, 8182 (2015)
A.H. Kianfar, P. Dehghani, M.M. Momeni, Mater Sci: Mater Electron. 27, 3368 (2016)
M.H. Habibi, B. Karimi, Iran. J. Environ. Technol. 1, 31 (2015)
A.A. El-Bindary, A.Z. El-Sonbati, A.A. Al-Sarawy, K.S. Mohamed, M.A. Farid, Spectrochim. Acta A 136, 1842 (2015)
M.R. Fathi, A. Asfaram, A. Farhangi, Spectrochim. Acta A 135, 364 (2015)
A. Nouri, A. Fakhri, Spectrochim. Acta A 138, 563 (2015)
M.M. Momeni, Y. Ghayeb, J. Mol. Catal. A Chem. 417, 107 (2016)
M.M. Momeni, M. Mirhosseini, M. Chavoshi, A. Hakimizade, J. Mater. Sci.: Mater. Electron. 27, 3941 (2016)
M.M. Momeni, M. Mirhosseini, M. Chavoshi, Ceram. Int. 42, 9133 (2016)
A. Senthilraja, B. Subash, P. Dhatshanamurthi, M. Swaminathan, M. Shanthi, Spectrochim. Acta A 138, 31 (2015)
S.J. Shettleworth, Cognition, Evolution and Behavior, 2nd edn. (Oxford, New York, 2010)
W. Glaze, J.W. Kang, D.H. Chapin, J. Inter. Ozone Assoc. 9, 335 (1987)
D. Rajamanickam, P. Dhatshanamurthi, M. Shanthi, Spectrochim. Acta A 138, 489 (2015)
N. Habibi, Spectrochim. Acta A 136, 1450 (2015)
N. Habibi, Spectrochim. Acta A 131, 55 (2014)
N. Habibi, Spectrochim. Acta A 125, 359 (2014)
N. Habibi, B. Karimi, J. Ind. Eng. Chem. 20, 3033 (2014)
Y. Shao, J. Sun, L. Gao, J. Phys. Chem. C 113, 6566 (2009)
Y. Liang, H. Wang, J. Zhou, Y. Li, J. Wang, T. Regier, H. Dai, J. Am. Chem. Soc. 134, 3517 (2012)
J.J.-Y. Wang, P.Y. Kuang, N. Li, Z.Q. Liu, Y.Z. Su, S. Chen, Ceram. Int. (2015). doi:10.1016/j.ceramint.2015.03
Acknowledgments
The authors wish to thank the University of Isfahan for financially supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habibi, M.H., Bagheri, P. Enhanced photo-catalytic degradation of naphthol blue black on nano-structure MnCo2O4: charge separation of the photo-generated electron–hole pair. J Mater Sci: Mater Electron 28, 289–294 (2017). https://doi.org/10.1007/s10854-016-5523-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5523-0