Abstract
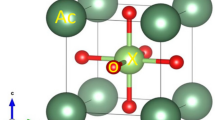
In this paper, structural refinement, Raman spectroscopy, optical and electrical properties of barium strontium molybdate [(Ba1−x Sr x )MoO4] ceramics with different (x) contents (x = 0; 0.1; 0.2; 0.3; 0.4; 0.5; 0.6; 0.7; 0.8; 0.9; and 1) were synthesized by the solid state reaction method. These ceramics were structurally characterized by X-ray diffraction (XRD), Rietveld refinement, and micro-Raman spectroscopy. The shape of the grains for these ceramics was observed by means of scanning electron microscopy (SEM) images. The optical properties were investigated using ultraviolet–visible (UV–Vis) absorption spectroscopy and photoluminescence (PL) measurements. The dielectric and ferroelectric properties were analyzed by permittivity (εr), loss tangent (tan δ) and polarization versus electric field (P–E) hysteresis loop. XRD patterns, Rietveld refinement, and micro-Raman spectra showed that all ceramics are monophasic with a scheelite-type tetragonal structure. A decreased of lattice parameters and unit cell volume was observed with the increase of Sr2+ ions into BaMoO4 lattice. Rietveld data were employed to model the [BaO8], [SrO8] and [MoO4] clusters in the tetragonal lattices. The SEM images indicate that increased x content promotes a decrease in the grain size and modifications in the shape. UV–Vis spectra indicated a decrease in the optical band gap values with an increase in x content in the (Ba1−x Sr x )MoO4 ceramics. PL emissions exhibit a non-linear behavior to increase or decrease with the increase of Sr2+ ions in the tetragonal lattices, when excited by a wavelength of 350 nm. The P–E decreases along with slim hysteresis loop towards higher Sr2+ ions concentration. These effects are correlated with decrease in lattice parameters and c/a ratio in this tetragonal lattice. The microwave dielectric constant and quality factor were measured using the method proposed by Hakki–Coleman. Temperature coefficient and quality factor of these materials were measured by vector network analyzer.










Similar content being viewed by others
References
G. Davidson, Spectroscopic Properties of Inorganic and Organometallic Compounds (Royal Society of Chemistry, Great Britain, 1998), pp. 1–303
L.S. Cavalcante, F.M.C. Batista, M.A.P. Almeida, A.C. Rabelo, I.C. Nogueira, N.C. Batista, J.A. Varela, M.R.M.C. Santos, E. Longo, M. Li, Structural refinement, growth process, photoluminescence and photocatalytic properties of (Ba1−x Pr2x/3)WO4 crystals synthesized by the coprecipitation method. RSC Adv. 2, 6438–6454 (2012)
R.C. Pullar, S. Farrah, N.M. Alford, MgWO4, ZnWO4, NiWO4 and CoWO4 microwave dielectric ceramics. J. Eur. Ceram. Soc. 27, 1059–1063 (2007)
I.L.V. Rosa, A.P.A. Marques, M.T.S. Tanaka, D.M.A. Melo, E.R. Leite, E. Longo, J.A. Varela, Synthesis, characterization and photophysical properties of Eu3+ doped in BaMoO4. J. Fluoresc. 18, 239–245 (2008)
A.J. Peter, I.B.S. Banu, Synthesis and luminescent properties of Tb3+ activated AWO4 based (A=Ca and Sr) efficient green emitting phosphors. J. Mater. Sci. Mater. Electron. 25, 2771–2779 (2014)
R. Sundaram, K. Nagaraja, Electrical and humidity sensing properties of lead (II) tungstate–tungsten (VI) oxide and zinc(II) tungstate–tungsten(VI) oxide composites. Mater. Res. Bull. 39, 581–590 (2004)
L. You, Y. Cao, Y.F. Sun, P. Sun, T. Zhang, Y. Du, G.Y. Lu, Humidity sensing properties of nanocrystalline ZnWO4 with porous structures. Sensors Actuators B Chem. 161, 799–804 (2012)
D.W. Kim, I.-S. Cho, S.S. Shin, S. Lee, T.H. Noh, D.H. Kim, H.S. Jung, K.S. Hong, Electronic band structures and photovoltaic properties of MWO4 (M=Zn, Mg, Ca, Sr) compounds. J. Solid State Chem. 184, 2103–2107 (2011)
M. Gancheva, A. Naydenov, R. Iordanova, D. Nihtianova, P. Stefanov, Mechanochemically assisted solid state synthesis, characterization, and catalytic properties of MgWO4. J. Mater. Sci. 50, 3447–3456 (2015)
L.S. Cavalcante, E. Moraes, M.A.P. Almeida, C.J. Dalmaschio, N.C. Batista, J.A. Varela, E. Longo, M. Siu Li, J. Andrés, A. Beltrán, A combined theoretical and experimental study of electronic structure and optical properties of β-ZnMoO4 microcrystals. Polyhedron 54, 13–25 (2013)
S.M.M. Zawawi, R. Yahya, A. Hassan, H.N.M.E. Mahmud, M.N. Daud, Structural and optical characterization of metal tungstates (MWO4; M=Ni, Ba, Bi) synthesized by a sucrose-templated method. Chem. Cent. J. 7, 80–89 (2013)
M.R.D. Bomio, L.S. Cavalcante, M.A.P. Almeida, R.L. Tranquilin, N.C. Batista, P.S. Pizani, M. Siu Li, J. Andres, E. Longo, Structural refinement, growth mechanism, infrared/Raman spectroscopies and photoluminescence properties of PbMoO4 crystals. Polyhedron 50, 532–545 (2013)
M.A.P. Almeida, L.S. Cavalcante, C. Morilla-Santos, C.J. Dalmaschio, S. Rajagopal, M. Siu Li, E. Longo, Effect of partial preferential orientation and distortions in octahedral clusters on the photoluminescence properties of FeWO4 nanocrystals. Cryst. Eng. Commun. 14, 7127–7132 (2012)
M.M.J. Sadiq, A.S. Nesaraj, Soft chemical synthesis and characterization of BaWO4 nanoparticles for photocatalytic removal of Rhodamine B present in water sample. J. Nanostruct. Chem. 5, 45–54 (2015)
X. Liu, L. Li, H.M. Noh, J.H. Jeong, K. Jang, D.S. Shin, Controllable synthesis of uniform CaMoO4:Eu 3+, M+(M= Li, Na, K) microspheres and optimum luminescence properties. RSC Adv. 5, 9441–9454 (2015)
V.S. Marques, L.S. Cavalcante, J.C. Sczancoski, A.F.P. Alcântara, M.O. Orlandi, E. Moraes, E. Longo, J.A. Varela, M. Siu Li, M.R.M.C. Santos, Effect of different solvent ratios (water/ethylene glycol) on the growth process of CaMoO4 crystals and their optical properties. Cryst. Growth Des. 10, 4752–4768 (2010)
J. Guo, D. Zhou, L. Wang, H. Wang, T. Shao, Z.M. Qi, X. Yao, Infrared spectra, Raman spectra, microwave dielectric properties and simulation for effective permittivity of temperature stable ceramics AMoO4–TiO2 (A= Ca, Sr). Dalton Trans. 42, 1483–1491 (2013)
A. Priya, E. Sinha, S.K. Rout, Structural, optical and microwave dielectric properties of Ba1−x Sr x WO4 ceramics prepared by solid state reaction route. Solid State Sci. 20, 40–45 (2013)
V.D. Araújo, R.L. Tranquilin, F.V. Motta, C.A. Paskocimas, M.I.B. Bernardi, L.S. Cavalcante, J. Andres, E. Longo, M.R.D. Bomio, Effect of polyvinyl alcohol on the shape, photoluminescence and photocatalytic properties of PbMoO4 microcrystals. Mater. Sci. Semicond. Process. 26, 425–430 (2014)
L.S. Cavalcante, V.M. Longo, J.C. Sczancoski, M.A.P. Almeida, A.A. Batista, J.A. Varela, M.O. Orlandi, E. Longo, M. Siu Li, Electronic structure, growth mechanism and photoluminescence of CaWO4 crystals”. Cryst. Eng. Commun. 14, 853–868 (2012)
B.P. Singh, R.A. Singh, Color tuning in thermally stable Sm3+ activated CaWO4 nanophosphors. New J. Chem. (2015). doi:10.1039/C4NJ01911C
M. Guzik, E. Tomaszewicz, S.M. Kaczmarek, J. Cybińska, H. Fuks, Spectroscopic investigations of Cd0.25Gd0.50□0.25WO4:Eu3+—a new promising red phosphor. J. Non-Cryst. Solids 356, 1902–1907 (2010)
A.F. Gouveia, J.C. Sczancoski, M.M. Ferrer, A.S. Lima, M.R.M.C. Santos, M. Li, R.S. Santos, E. Longo, L.S. Cavalcante, Experimental and theoretical investigations of electronic structure and photoluminescence properties of β-Ag2MoO4 microcrystals. Inorg. Chem. 53, 5589–5599 (2014)
A. Dias, Theoretical calculations and hydrothermal processing of bawo4 materials under environmentally friendly conditions. J. Solut. Chem. 40, 1126–1139 (2011)
Y. Guo, G. Fan, Z. Huang, J. Sun, L. Wang, T. Wang, J. Chen, Determination of standard molar enthalpies of formation of SrMoO4 micro/nano structures. Thermochim. Acta 530, 116–119 (2012)
G. Bayer, H.-G. Wiedemann, Formation of scheelite (CaWO4) and powellite (CaMoO4) by displacement reactions. Thermochim. Acta 133, 125–130 (1988)
C. Bouzidi, N. Sdiri, A. Boukhachem, H. Elhouichet, M. Féri, Impedance analysis of BaMo1−x W x O4 ceramics. Superlattices Microstruct. 82, 559–573 (2015)
X. He, M. Guan, Z. Li, T. Shang, N. Lian, Q. Zhou, Enhancement of fluorescence from BaMoO4:Pr3+ deep-red-emitting phosphor via codoping Li+ and Na+ ions. J. Am. Ceram. Soc. 94, 2483–2488 (2011)
B.K. Maji, H. Jena, K.V.G. Kutty, Effect of la-substitution on the electrical conductivity of Sr1−x La x MoO4+δ (x = 0 − 0.3) compounds. J. Mater. Eng. Perform. 23, 3126–3132 (2014)
R. Cao, K. Chen, Q. Hu, W. Li, H. Ao, C. Cao, T. Liang, Synthesis and luminescence properties of Sr(1x−y−z)MoO4:xEu3+, yBi3+, zR+ (R+=Li+, Na+, and K+) phosphors. Adv. Powder Technol. 26, 500–504 (2015)
Y.-S. Cho, Y.-D. Huh, Preparation and optical properties of green-emitting BaMoO4:Tb3+, Na+ nanophosphors for transparent displays. Electron. Mater. Lett. 10, 1185–1189 (2014)
M. Lei, C.X. Ye, S.S. Ding, K. Bi, H. Xiao, Z.B. Sun, D.Y. Fan, H.J. Yang, Y.G. Wang, Controllable route to barium molybdate crystal and their photoluminescence. J. Alloys Compd. 639, 102–105 (2015)
J. Diaz-Algara, J.C. Rendón-Angeles, Z. Matamoros-Veloza, K. Yanagisawa, J.L. Rodriguez-Galicia, J.M. Rivera-Cobo, Single-step synthesis of SrMoO4 particles from SrSO4 and their anti-corrosive activity. J. Alloys Compd. 607, 73–84 (2014)
G. Xing, Y. Li, Y. Li, Z. Wu, P. Sun, Y. Wang, C. Zhao, G. Wu, Morphology-controllable synthesis of SrMoO4 hierarchical crystallites via a simple precipitation method. Mater. Chem. Phys. 127, 465–470 (2011)
C. Zhang, L. Zhang, C. Song, G. Jia, S. Huo, S. Shen, Well-defined barium molybdate hierarchical architectures with different morphologies: controllable synthesis, formation process, and luminescence properties. J. Alloys Compd. 589, 185–191 (2014)
K.K. Aruna, R. Manoharan, Electrochemical hydrogen evolution catalyzed by SrMoO4 spindle particles in acid water. Int. J. Hydrog. Energy 38, 12695–12703 (2013)
A.P.A. Marques, D.M.A. de Melo, E. Longo, C.A. Paskocimas, P.S. Pizani, E.R. Leite, Photoluminescence properties of BaMoO4 amorphous thin films. J. Solid State Chem. 178, 2346–2353 (2005)
A.P.A. Marques, M.T.S. Tanaka, E. Longo, E.R. Leite, I.L.V. Rosa, The role of the Eu3+ concentration on the SrMoO4: Eu phosphor properties synthesis characterization and photophysical studies. J. Fluoresc. 21, 893–899 (2011)
L.S. Cavalcante, J.C. Sczancoski, R.L. Tranquilin, J.A. Varela, E. Longo, M.O. Orlandi, Growth mechanism of octahedron-like BaMoO4 microcrystals processed in microwave-hydrothermal: Experimental observations and computational modeling. Particuology 7, 353–362 (2009)
S. Wannapop, T. Thongtem, S. Thongtem, Characterization of donut-like SrMoO4 produced by microwave-hydrothermal process. J. Nanomater. 2013, 474576–474581 (2013)
Z. Li, J. Du, J. Zhang, T. Mu, Y. Gao, B. Han, J. Chen, J. Chen, Synthesis of single crystal BaMoO4 nanofibers in CTAB reverse microemulsions. Mater. Lett. 59, 64–68 (2005)
Q. Gong, X. Qian, X. Ma, Z. Zhu, Large-scale fabrication of novel hierarchical 3D CaMoO4 and SrMoO4 mesocrystals via a microemulsion-mediated route. Cryst. Growth Des. 6, 1821–1825 (2006)
M. Yoshimura, M. Ohmura, W.-S. Cho, M. Yashima, M. Kakihana, Preparation and luminescence of crystallized Ba1−x Sr x MoO4 solid-solution films by an electrochemical method at room temperature. Jpn. J. Appl. Phys. 36, L1229–L1231 (1997)
C.-T. Xia, V.M. Fuenzalida, R.A. Zarate, Electrochemical preparation of crystallized Ba1−x Sr x MoO4 solid-solution films at room-temperature. J. Alloys Compd. 316, 250–255 (2001)
D.J. Gao, D.Q. Xiao, J. Bi, P. Yu, W. Zhang, G.L. Yu, J.G. Zhu, Electrochemical deposition of Ba1−x Sr x MoO4 thin films at room temperature. MRS Proceedings 755, DD6.3 (2002)
M. Sahu, K. Krishnan, B.K. Nagar, D. Jain, M.K. Saxena, C.G.S. Pillai, S. Dash, Characterization and thermo physical property investigations on Ba1−x Sr x MoO4 (x = 0, 0.18, 0.38, 0.60, 0.81, 1) solid-solutions. J. Nucl. Mater. 427, 323–332 (2012)
I.C. Nogueira, L.S. Cavalcante, P.F.S. Pereira, M.M. de Jesus, J.M. Rivas Mercury, N.C. Batista, M.S. Li, E. Longo, Rietveld refinement, morphology and optical properties of (Ba1−x Sr x )MoO4 crystals. J. Appl. Cryst. 46, 1434–1446 (2013)
V. Nassif, R.E. Carbonio, J.A. Alonso, Neutron diffraction study of the crystal structure of BaMoO4: a suitable precursor for metallic BaMoO3 perovskite. J. Solid State Chem. 146, 266–270 (1999)
C. Bernuy-Lopez, M. Allix, C.A. Bridges, J.B. Claridge, M.J. Rosseinsky, Sr2MgMoO6−δ: structure, phase stability, and cation site order control of reduction. Chem. Mater. 19, 1035–1043 (2007)
B.H. Toby, EXPGUI, a graphical use interphase for GSAS. J. Appl. Crystallogr. 34, 210–221 (2001)
J.C. Sczancoski, L.S. Cavalcante, N.L. Marana, R.O. da Silva, R.L. Tranquilin, M.R. Joya, P.S. Pizani, J.A. Varela, J.R. Sambrano, M. Siu Li, E. Longo, J. Andrés, Electronic structure and optical properties of BaMoO4 powders. Curr. Appl. Phys. 10, 614–624 (2010)
A.L. Patterson, The Scherrer formula for X-ray particle size determination. Phys. Rev. 56, 978–982 (1993)
K. Momma, F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 44, 1272–1276 (2011)
K. Momma, F. Izumi, VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Cryst. 41, 653–658 (2008)
P.F.S. Pereira, I.C. Nogueira, E. Longo, E.J. Nassar, I.L.V. Rosa, L.S. Cavalcante, Rietveld refinement and optical properties of SrWO4:Eu3+ powders prepared by the non-hydrolytic sol-gel method. J. Rare Earths 33, 113–128 (2015)
R.F. Gonçalves, L.S. Cavalcante, I.C. Nogueira, E. Longo, M.J. Godinho, J.C. Sczancoski, V.R. Mastelaro, I.M. Pinatti, I.L.V. Rosa, A.P.A. Marques, Rietveld refinement, cluster modelling, growth mechanism and photoluminescence properties of CaWO4:Eu3+ microcrystals. Cryst. Eng. Commun. 17, 1654–1666 (2015)
T.T. Basiev, A.A. Sobol, Y.K. Voronko, P.G. Zverev, Spontaneous Raman spectroscopy of tungstate and molybdate crystals for Raman lasers. Opt. Mater. 15, 205–216 (2000)
J.C. Sczancoski, L.S. Cavalcante, M.R. Joya, J.A. Varela, P.S. Pizani, E. Longo, SrMoO4 powders processed in microwave-hydrothermal: Synthesis, characterization and optical properties. Chem. Eng. J. 140, 632–637 (2008)
T.T. Basiev, A.A. Sobol, P.G. Zverev, L.I. Ivleva, V.V. Osiko, R.C. Powell, Raman spectroscopy of crystals for stimulated Raman scattering. Opt. Mater. 11, 307–314 (1999)
Q. Gong, X. Qian, H. Cao, W. Du, X. Ma, M. Mo, Novel shape evolution of BaMoO4 microcrystals. J. Phys. Chem. B. 110, 19295–19299 (2006)
S.P. Culver, F.A. Rabuffetti, S. Zhou, M. Mecklenburg, Y. Song, B.C. Melot, R.L. Brutchey, Low-temperature synthesis of AMoO4 (A=Ca, Sr, Ba) scheelite nanocrystals. Chem. Mater. 25, 4129–4134 (2013)
P. Kubelka, F. Munk-Aussig, Ein beitrag zur optik der farbanstriche. Zeit. Fur. Tech. Physik. 12, 593–601 (1931)
M.L. Myrick, M.N. Simcock, M. Baranowski, H. Brooke, S.L. Morgan, J.N. Mccutcheon, The Kubelka-Munk diffuse reflectance formula revisited. Appl. Spectrosc. Rev. 46, 140–165 (2011)
R.A. Smith, Semiconductors, 2nd edn. (Cambridge University Press, London, 1978), p. 434
D. Errandonea, L. Gracia, R. Lacomba-Perales, A. Polian, J.C. Chervin, Compression of scheelite-type SrMoO4 under quasi-hydrostatic conditions: redefining the high-pressure structural sequence. J. Appl. Phys. 113, 123510–123519 (2013)
R. Vali, Electronic properties and phonon spectra of SrMoO4. Comput. Mater. Sci. 50, 2683–2687 (2011)
E. Longo, D.P. Volanti, V.M. Longo, L. Gracia, I.C. Nogueira, M.A.P. Almeida, A.N. Pinheiro, M.M. Ferrer, L.S. Cavalcante, J. Andres, Toward an understanding of the growth of Ag filaments on α-Ag2WO4 and their photoluminescent properties: a Combined experimental and theoretical study. J. Phys. Chem. C 118, 1229–1239 (2014)
L. Gracia, V.M. Longo, L.S. Cavalcante, A. Beltrán, W. Avansi, M.S. Li, V.R. Mastelaro, J.A. Varela, E. Longo, J. Andre, Presence of excited electronic state in CaWO4 crystals provoked by a tetrahedral distortion: an experimental and theoretical investigation. J. Appl. Phys. 110, 043501–043511 (2011)
A.P. de Moura, L.H. de Oliveira, I.L.V. Rosa, C.S. Xavier, P.N. Lisboa-Filho, M.S. Li, F.A. La Porta, E. Longo, J.A. Varela, Structural, optical, and magnetic properties of NiMoO4 nanorods prepared by microwave sintering. Sci. World J. 2015, 315084–315091 (2015)
M.R.D. Bomio, R.L. Tranquilin, F.V. Motta, C.A. Paskocimas, R.M. Nascimento, L. Gracia, J. Andres, E. Longo, Toward understanding the photocatalytic activity of PbMoO4 powders with predominant (111), (100), (011), and (110) facets: a combined experimental and theoretical study. J. Phys. Chem. C 117, 21382–21395 (2013)
J. Zhang, L. Li, W. Zi, L. Zou, S. Gan, G. Ji, Size-tailored synthesis and luminescent properties of three-dimensional BaMoO4, BaMoO4:Eu3+ micron-octahedrons and micron-flowers via sonochemical route. Luminescence 30, 280–289 (2015)
S.S. Ding, M. Lei, H. Xiao, G. Liu, Y.C. Zhang, K. Huang, C. Liang, Y.J. Wang, R. Zhang, D.Y. Fan, H.J. Yang, Y.G. Wang, Morphology evolution and photoluminescence of barium molybdate controlled by poly (sodium-4-styrenesulfonate). J. Alloys Compd. 579, 549–552 (2013)
L. Li, R. Li, W. Zi, S. Gan, Hydrothermal synthesis and luminescent properties of color-tunable Dy3+ doped and Eu3+/Tb3+ co-doped MMoO4 (M=Ca, Sr, Ba) phosphors. Phys. B 458, 8–17 (2015)
P. Jena, S.K. Gupta, V. Natarajan, M. Sahu, N. Satyanarayana, M. Venkateswarlu, Structural characterization and photoluminescence properties of sol–gel derived nanocrystalline BaMoO4:Dy3+. J. Lumin. 158, 203–210 (2015)
S. Cho, Synthesis and luminescence properties of SrMoO4:RE3+ (RE=Eu or Tb) phosphors. J. Korean Phys. Soc. 64, 1529–1534 (2014)
J. Zhang, R. Li, L. Liu, L. Li, L. Zou, S. Gan, G. Ji, Self-assembled 3D sphere-like SrMoO4 and SrMoO4:Ln3+ (Ln=Eu, Sm, Tb, Dy) microarchitectures: Facile sonochemical synthesis and optical properties. Ultrason. Sonochem. 21, 1736–1744 (2014)
C. Shivakumara, R. Saraf, Eu3+ activated SrMoO4 phosphors for white LEDs applications: Synthesis and structural characterization. Opt. Mater. 42, 178–186 (2015)
L. Li, Z. Leng, W. Zi, S. Gan, Hydrothermal synthesis of SrMoO4:Eu3+, Sm3+ phosphors and their enhanced luminescent properties through energy transfer. J. Electron. Mater. 43, 2588–2596 (2014)
V.M. Longo, L.S. Cavalcante, E.C. Paris, J.C. Sczancoski, P.S. Pizani, M. Siu Li, J. Andrés, E. Longo, J.A. Varela, Hierarchical assembly of CaMoO4 nano-octahedrons and their photoluminescence properties. J. Phys. Chem. C 115, 5207–5219 (2011)
Z. Xia, D. Chen, Synthesis and luminescence properties of BaMoO4:Sm3+ phosphors. J. Am. Ceram. Soc. 1401, 1397–1401 (2010)
A.P. de Azevedo Marques, D.M.A. De Melo, C.A. Paskocimas, P.S. Pizani, M.R. Joya, E.R. Leite, E. Longo, Photoluminescent BaMoO4 nanopowders prepared by complex polymerization method (CPM). J. Solid State Chem. 179, 671–678 (2006)
R.T. Lam, G. Blasse, Luminescence of barium molybdate (BaMoO4). J. Chem. Phys. 71, 3549 (1979)
L.S. Cavalcante, M.A.P. Almeida, W. Avansi Jr, R.L. Tranquilin, E. Longo, N.C. Batista, V.R. Mastelaro, M. Siu Li, Cluster coordination and photoluminescence properties of α-Ag2WO4 microcrystals. Inorg. Chem. 51, 10675–10687 (2012)
P. Jena, S.K. Gupta, V. Natarajan, O. Padmaraj, N. Satyanarayana, M. Venkateswarlu, On the photo-luminescence properties of sol–gel derived undoped and Dy3+ ion doped nanocrystalline scheelite type AMoO4 (A=Ca, Sr and Ba). Mater. Res. Bull. 64, 223–232 (2015)
L. Wei, Y. Liu, Y. Lu, T. Wu, Morphology and Photoluminescence of Ba0.5Sr0.5MoO4 powders by a molten salt method. J. Nanomater. 2012, 398582–398587 (2012)
E. Ryu, Y. Huh, Synthesis of hierarchical self-assembled BaMoO4 microcrystals. Bull. Korean Chem. Soc. 29(2), 503–506 (2008)
C. Mao, J. Geng, X. Wu, J. Zhu, Selective synthesis and luminescence properties of self-assembled SrMoO4 superstructures via a facile sonochemical route. J. Phys. Chem. C 114, 1982–1988 (2010)
S. Devi, A.K. Jha, Structural, dielectric and ferroelectric properties of tungsten substituted barium titanate ceramics. Asian J. Chem. 21, S117–S124 (2009)
L. Jin, F. Li, S. Zhang, Decoding the fingerprint of ferroelectric loops: comprehension of the material properties and structures. J. Am. Ceram. Soc. 97, 1–27 (2014)
G.H. Haertling, Ferroelectric ceramics: history and technology. J. Am. Ceram. Soc. 82, 797–818 (1999)
K.M. Rabe, M. Dawber, Modern physics of ferroelectrics: essential background. Appl. Phys. 105, 1–30 (2007)
P.D. Hakki, B.W. Coleman, A dielectric resonator method of measuring inductive capacities in the millimeter range. IRE Trans. Microw. Theory Tech. 8, 402–410 (1960)
R.D. Shannon, Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 73(1), 348 (1993)
S.D. Ramarao, S.R. Kiran, V.R.K. Murthy, Structural, lattice vibrational, optical and microwave dielectric studies on Ca1−x Sr x MoO4 ceramics with scheelite structure. Mater. Res. Bull. 56, 71–79 (2014)
S. Kobayashi, Y. Tanaka, Resonant modes of a dielectric rod resonator short-circuited at both ends by parallel conducting plates. IEEE Trans. Microw. Theory Techol. 28, 1077–1085 (1980)
G.K. Choi, J.R. Kim, S.H. Yoon, K.S. Hong, Microwave dielectric properties of scheelite (A = Ca, Sr, Ba) and wolframite (A=Mg, Zn, Mn) AMoO4 compounds. J. Eur. Ceram. Soc. 27, 3063–3067 (2007)
S.H. Yoon, D.W. Kim, S.Y. Cho, K.S. Hong, Investigation of the relations between structure and microwave dielectric properties of divalent metal tungstate compounds. J. Eur. Ceram. Soc. 26, 2051–2054 (2006)
D.A. Durilin, O.V. Ovchar, A.G. Belous, Effect of nonstoichiometry on the structure and microwave dielectric properties of Ba1−x (Zn1/2W1/2)O3−x and Ba(Zn1/2−y W1/2)O3−y/2. Inorg. Mater. 47, 313–316 (2011)
Acknowledgments
Indian authors gratefully acknowledge the financial support from DST Fast Track project (F. No. SB/FT/CS-044/2013) Govt. of India. The Brazilian authors acknowledge the financial support of agencies: CNPq (304531/2013-8) and FAPESP (2012/14004-5).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ghosh, S.K., Rout, S.K., Tiwari, A. et al. Structural refinement, Raman spectroscopy, optical and electrical properties of (Ba1−xSrx)MoO4 ceramics. J Mater Sci: Mater Electron 26, 8319–8335 (2015). https://doi.org/10.1007/s10854-015-3498-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3498-x