Abstract
Mg doped SnO thin films were fabricated via electron beam evaporator on the glass substrate. The XRD analysis showed that reference and Mg doped SnO thin films were consisted of tetragonal α-SnO phase with preferred directions along (101) and (002) orientations. It was observed that the intensity of the diffraction pattern peaks increased and crystallite size decreased with the Mg doping. Scanning electron microscopy showed that the needle-like particles having length in the range of 0.4–0.6 μm with a diameter of 0.1–0.2 μm are sintered together to form a compact structure of Sn1−xMgxO layer. In the Raman spectrum, two active mode (A 2u and E u ) were observed for Sn1−xMgxO thin films. The complex plot (Nyquist plot) showed the data point laying on a single semicircle and the dc resistance increases with the increase of Mg doping concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Commonly observed phases of tin oxide are rutile structure for tin dioxide (SnO2) and litharge structure for tin monoxide (SnO), corresponding to the Sn oxidation states of +4 and +2, respectively. As far as from the literature concerned, there have been carried out lot of research work to enlighten the physical and chemical properties of rutile phase of tin oxide (SnO2) [1, 2]. Some of the most common applications of SnO2 are: gas sensor [1, 3], dye-based solar cell [4], oxidation catalyst [5], transparent conductor [6], and photo conductor [7]. Whereas, few reports has been cited to investigate the micro-structural and electrical properties of SnO [8, 9]. The SnO has been marked as anode material [10] and has been employed for other applications such as for gas sensitive material [11], coating substance [12], catalyst [12], precursor for the production of tin dioxide [13],and for p-channel thin film transistor [14, 15]. The reported crystal habit of SnO is tetragonal crystal structure which is similar to that of the ‘red’ (α or litharge) modification of isovalent PbO [16]. The tetragonal crystal structure of SnO is layered with an AA Stacking sequence of slabs. Each slab consists of square planar arrangement of oxygen atoms (O–O = 2.69 Å), sandwiched between sub layers of tin ions. Tin ions form four square pyramidal bonds with bond length in the range of 2.22 Å with oxygen atoms [17]. The electronic band gap of SnO phase lies between 2.5 and 3 eV at room temperature, which is smaller to phase [SnO2 (3.6 eV)]. The existence of different oxidation states in tin ion makes it more favorable to have non-stoichiometric tin oxide phases [18, 19].
In the literature, few reports have been cited yet for discussing the effect of doping alkaline earth metals (Mg) on the structure, optical and electrical properties of tin monoxide (SnO). Liang et al. [20] have reported that increase in the concentration of Y doping in the SnO, the deterioration of crystallinity and increase in optical band gap of SnO has been observed. Gua et al. [21] speculated that the charge defect complex act as the accepter when large size do-pant such as Sb has been introduced in SnO crystal structure.
Herein, we have reported the deposition of alkaline earth metal (Mg) doped SnO thin films Sn1−xMgxO (where x = 0–0.1) by electron beam evaporator. The deposited thin films have been characterized to investigate the microstructural, optical and electrical properties. The crystallinity and phase purity of the Sn1−xMgxO thin films have been analyzed by X-ray diffractometer (XRD), and the microstructure has been studied by scanning electron microscopy (SEM) and Raman spectroscopy. The electrical conductivity, carrier mobility and concentrations have been measured using impedance spectroscopy.
2 Experimental details
Sn1−xMgxO thin films were deposited on BK-7 glass substrate by electron beam evaporator. The glass substrate was washed before deposition, with acetone/de-ionized H2O ultrasonically and subsequently dried. Quantitative amount of both the reactants (Mg turning and SnO2) of required concentration were placed inside the deposition chamber on sample holder and mixed by sintering. The deposition chamber was evacuated to 1 × 10−5mbar and maintained at oxygen pressure of 1 × 10−4mbar. Prior to the deposition, the substrate was heated to 250 °C for 30 min. The reactants were evaporated to deposit thin films on the surface of substrate from the source at 250 °C. The films thickness and rate of deposition were monitored by quartz crystal oscillator and kept at around 200 nm and 0.10 nm/s, respectively.
The crystallinity and phase purity of the deposited MgxSn1−xO thin films were analyzed by X-ray diffraction patterns using multipurpose X-ray differctometter (Bruker, D8 Advance) with Cu–Kα source radiation. Impedance spectroscopic (IS) measurements were made at room temperature using Alpha-A High Performance Frequency Analyzer, Novo-control Technologies, Germany in the frequency range from 0.1 Hz to 1 MHz. Silver paint electrodes were used at a separation of about 15 mm; while the width of the samples was about 10 mm. The contacts were allowed to dry for 24 h in air. Raman measurements were performed by using PL/Raman Photon system USA [Ne-Cu laser (948 nm)]. The reflectance of the pure and Mg doped SnO thin films were recorded at room temperature by a Perkin Elmer UV/VIS/NIR Lambda 19 spectrophotometer in the wavelength range 200–3,000 nm. The surface morphology and elemental characterization of the deposited thin films were analyzed using a high resolution Scanning Electron Microscope (SEM, NNL 200) coupled with an energy dispersive X-ray spectrometer (EDX, GENESIS).
3 Result and discussion
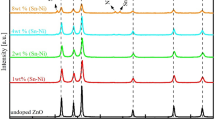
Non-destructive X-ray diffraction analysis was used to investigate the structural and crystallanity of the of Sn1−xMgxO thin films deposited by electron beam evaporator, shown in Fig. 1. From the X-ray analysis, it has been revealed that the entire sample showed typical peaks of α-SnO without any detectable dopant related peaks, rather than the increase in peak sharpness intensity with the increase of dopant concentration has been observed. This effect may be explain on the bases that dopant is well dispersed and went either into interstitial position or substitution sites of α-SnO crystal structure. The reference thin film (SnO) has been matched to α-SnO tetragonal phase with lattice parameters a = b = 3.802 Å and c = 4.386 Å [JCPDS card 006-0395]. In the X-ray diffraction pattern, the intensity of tetragonal phase increases with the increase of Mg doping concentration. The preferred growth orientations in the XRD pattern are (101), (002) and (112), an increase of Mg doping result increase in sharpness of peak intensity which may be attributed due to elongation of tetragonal phase. Both the Mg2+ (0.67 nm) and Sn2+ (0.88 nm) has very close ionic radii, which may be a important factor for no change in the value of lattice parameters calculated for pure and doped SnO thin films.
The average crystallite size was calculated by using Scherer formula, based on the full width half maxima of the XRD peak (002) of each sample [22].
where θ is the Bragg’s angle, λ = 1.54056 Å and β 2 = (FWHM)2 − b 2 is the wavelength of Cu–Kα radiation, b is the factor of compensate for the line broadening effect, D is the average diameter of the Crystallite, and K is the shape factor which is approximately unity. The line broadening factor (b) is estimated with powder sample of silicon. The crystallite size varies from 76 nm for reference thin film to 23 nm for Mg doped SnO thin films, respectively. It has been observed that by increasing the dopant concentration, the crystallite size of Mg doped SnO thin films decreases with increasing Mg content.
Morphological and surface studies of pure and Mg-doped SnO thin films fabricated by electron beam evaporation have been carried out using scanning electron microscopy (SEM) shown in Fig. 2. It is evident from the Fig. 2 that the grains of pure and the Mg-doped SnO thin films possess small needle-like symmetry. The length and diameter of each needle is in the range of 0.4–0.6 and 0.1–0.2 μm, respectively. SEM study reveals that each needle like grains has been formed as a result of incorporation and agglomeration of crystallite (~25 nm) and each needle are fused with each other forming an interconnected 3D network with electronic connections. Upon increasing the concentration of Mg doping, the surface morphology and roughness of the thin films changes and the grains size decreases. Thus SEM studies corroborate the XRD findings, where the crystallite size decreases with the increase of Mg doping. The preferred orientation of thin films with the morphology of characteristic needle shaped grains shown along (101) and (002) plane, respectively.
Mg content in the deposited thin films was calculated by quantitative EDX analysis shown in Fig. 3 and also confirms the retention of same metallic ratio in the deposited Sn1−xMgxO (0 ≤ x ≤ 0.1) thin films for Sn, Mg and O respectively. No other elements except oxygen were detected in the EDX spectra, which assures the uniform distribution of constituent metals as in the Sn1−xMgxO (0 ≤ x ≤ 0.1) thin films and also confirmed the absence of impurities.
Raman spectroscopy is considered more powerful non-destructive technique for study the crystalline quality, structure disorder and defects in doped semiconductor alloy. It gives important information about the local structure changes due to incorporation of highly mismatching of Mg ions into SnO host lattice. The α-SnO optic modes have the following irreducible representation. [23].
The symbols A and B are the non-degenerated vibrational modes and E doubly degenerated modes. The A 1g , B 1g and \( \left( {E_{g}^{1} \;{\text{and}}\;E_{g}^{2} } \right) \) are the Raman active modes and A2u and E u are the Raman and Infra-red active modes. From the Fig. 4, the Raman spectra of pure SnO thin films A 1g and \( E_{g}^{1} \) have been observed, while B 1g and \( E_{g}^{2} \) are not identify. The positions of the observed modes in pure SnO thin film spectrum are at 222.8 and 448 cm−1, close to the theoretical value [24]. With the Mg doping in SnO, the position of same modes were observed but slightly shifted to the larger wave number by increasing the Mg doping and the intensity of these modes increases as the Mg doping due to the slightly mismatched Mg2+ ion in SnO lattice.
Figure 5 shows the reflectance ‘R’ of pure and doped SnO thin films as the function of wave length. The spectra show the interference pattern in which doped SnO thin films spectra have almost similar trend with slightly shift of peak toward the lower wave length by increasing the doping. It may be due to the structure modification as observed in Raman analysis.
The refractive index can be determined by reflectance data using the following relation [25].
The refractive index (n) of pure and doped SnO thin films as the function of wave length is shown in Fig. 6, which show the oscillatory behavior of the refractive index (from nmin = 1.19 to nmax = 1.7) in UV, visible and near IR regions.
Impedance spectroscopy (IS) is a powerful method of characterization many of electrical properties of materials or films [26]. The great strength of IS is that, with the appropriate data analysis, it is often possible to characterize the electrically active regions in the material by describing their existence and their individual electrical behavior [27]. The basis of IS, is analysis the impedance of the sample under investigation based on the applied frequencies. The data obtain by IS measurement are often expressed in term of Nyquist plots i.e. Z’’ (imaginary part of the impedance) versus Z’ (real part of the impedance). The Nyquist plots for pure and doped SnO thin films are shown in Fig. 7. The IS plot of pure SnO thin film show one depress semicircle. The semicircle depressed due to the relaxation of time and therefore shows the non-Debye nature of the thin film [28]. While the IS plots of Mg doped SnO thin films show the semicircle. The tail observed at the lower frequencies is due to the electrode effect [28]. It may be notice that the electrical behavior of the SnO thin films is strongly dependent on the doping concentration of Mg.
The measured data is well fitted with simulated curve by using an equivalent RC circuit. The equivalent RC circuit, dc resistance by intercept on the real impedance axis in the Nyquist plot and the optimum value of capacitance are given in the Table 1. The capacitance CPE1 is found to be approximately same for pure and doped thin films. But the value of R1 is increases by increasing the doping level of Mg in SnO, It may be due to the decreasing of grain size by increasing the doping concentration.
4 Conclusions
Sn1−xMgxO (0 ≤ x ≤ 0.1) thin films have been fabricated by electron beam evaporator on BK-7 glass substrate. The microstructure and electrical properties have been characterized by X-ray diffraction, scanning electron microscopy, energy dispersive X-ray, Hall Effect measurement and impedance spectroscopy. The reference and Mg doped SnO thin films consist of tetragonal α-SnO phase with preferred directions along (101) and (002) orientation. The crystalline size increases from 35 to 63 nm with increasing the Mg doping. The two Raman active modes A 2u and E u are observed in Sn1−xMgxO thin films, which shift to higher wave number with Mg doping concentration. The SEM images show that the thin films consist of needle like particle with size in the range of from 0.4 to 0.6 μm and diameter 0.1–0.2 μm, respectively. The EDX analysis shows that the dopant concentration is in accordance with the quantity taken to deposit Sn1−xMgxO thin films. The dc resistance of reference SnO thin film increases by increasing the Mg doping. The simulated curve of Nyquist plots of Sn1−xMgxO thin films based on the circuit comprise on the parallel combination of resistor and capacitor. On the basis of structure and electrical properties, the deposited Sn1−xMgxO thin films are good for thin film transistor (TFT).
References
H. Jerger, F. Kohler, H.B. Becker, R. Keller, Seifert. Sens Actuators B 81(2), 301 (2002)
Y.S. Feng, R.S. Yao, L.D. Mang, Chin. Phys. Lett. 21(7), 1374 (2004)
Y. Zhang, A. Kolmakov, S. Chretien, H. Metiu, Nano Lett. 4(3), 403 (2004)
S. Ferrere, A. Zaban, B.A. Gsegg, J. Phys. Chem. B 101(23), 4490 (1997)
S. Wang, J. Huang, Y. Zhao, S. Wang, S. Wu, S. Zhang, W. Huang, Mater. Lett. 60(13), 1706 (2006)
R.E. Presley, C.L. Munses, C.H. Park, D. Hong, J.F. Wager, D.A. Keszler, J. Phys. D 37(20), 2810 (2004)
H. Zhang, J. Hanna, J. Appl. Phys. 88(1), 270 (2000)
S. Majumdar, S. Chakraborty, P.S. Devi, A. Sen, Mater. Lett. 62(8–9), 1249 (2008)
M.M. Rao, M. Jayalaksmi, B. Ramachandrareddy, S.S. Madhavevdra, M.L. Lantam, Chem. Lett. 34(5), 712 (2005)
J.J. Ning, T. Jiang, K.K. Men, Q.Q. Dai, D.M. Li, Y.J. Wei, B.B. Liu, J. Phys. Chem. C 113(32), 14140 (2009)
J. Calderer, P. Molinas, J. Llobet, X. Correig, Microelectron. Reliab. 40, 807 (2000)
H. Giefers, F. Porsch, G. Wortmann, Solid State Ion. 176(1–2), 199 (2005)
X.Q. Pan, L. Fu, J. Appl. Phys. 89(11), 6048 (2001)
Y. Ogo, H. Hiramatsu, K. Nomura, H. Yanagi, T. Kamiya, Appl. Phys. Lett. 93(21), 32113 (2008)
E. Fortunato, R. Barros, P. Barquinha, S.H.K. Park, Appl. Phys. Lett. 97(7), 52105 (2010)
R.W.G. Wyckoff, Crystal Structure, 2nd edn. (Wiley, New York, 1963), p. 359
X. Wang, F.X. Zhang, I. Loa, K. Syassen, M. Hanfland, Y.L. Mathis, Phys. State. Sol (b). 241(14), 3168 (2004)
J.G. Zhang, X. Pan, M. Schweizer, U. Weimar, W. Gopel, Philos. Mag. Lett. 73(6), 93 (1996)
Q.H. Wu, J. Song, J. Kang, Q.F. Dong, S.T. Wu, S.G. Sun, Mater. Lett. 61(17), 3679 (2007)
L.Y. Liang, Z.M. Liu, H.T. Cao, W.Y. Xu, J. Phys. D. 45(8), 85101 (2012)
W. Guo, L. Fu, Y. Zhang, K. Zhang, G. Graham, L.Y. Liang, Appl. Phys. Lett. 96(9), 42113 (2010)
H.P. Klug, L.E. Alexander, X-ray Diffraction Procedure for Polycrystalline and Amorphous Material, 2nd edn. (Wiley, New York, 1954), p. 491
Y.W. Li, Y. Li, T. Cui, L.J. Zhang, Y.M. Ma, G.T. Zou, J. Phys. Condens. Matter.19(42), 425230 (2007)
S. Koval, R. Burriel, M.G. Stachiotti, M. Castro, Phys. Rev. B 60(21), 14496 (1999)
A.O.-Flores, P.B.-Perez, R.C.-Rodriguez, A.I. Oliva, Revista Mexicana De Fisica 52(1) 15, (2006)
E. Barsoukov, J. Ross Macdonald (eds.), Impedance Spectroscopy, Theory, Experimental and Application. 2nd edn. (Wiley, Berlin, 2005), p. 41
R. West, D.C. Sinclair, N. Hirose, J. Electroceram. 1(1), 65 (1997)
A.C. Bose, D. Kalpana, P. Thangadurai, S. Ramasamy, J. Power Sources 107(1), 138 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ali, S.M., Muhammad, J., Hussain, S.T. et al. Study of microstructural, optical and electrical properties of Mg dopped SnO thin films. J Mater Sci: Mater Electron 24, 2432–2437 (2013). https://doi.org/10.1007/s10854-013-1114-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1114-5