Abstract
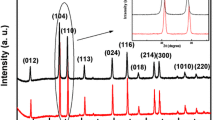
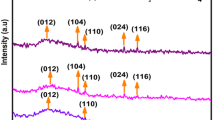
α- Fe2O3 nanoparticles have been synthesized by gel evaporation method in air at 300°C. The average size of as synthesized α-Fe2O3 nanoparticle was estimated to be 30 nm and the particles were of good crystalline nature. Shape of the nanoparticles were slightly deviated from spherical which is attributed to the asymmetric growth of primary nuclei. MicroRaman and X-ray diffraction results have shown mixed phases of α-Fe2O3 and γ-Fe2O3. However, the α-Fe2O3 phase is more predominant than γ-Fe2O3 due to the incomplete nucleation of α-Fe2O3 particles at the size of 30 nm. The vibrating sample magnetometer measurement shows that the nanoparticles possess ferromagnetic property.





Similar content being viewed by others
References
A. Navarotsky, L. Mazeina, J. Majzlan, Science 319, 1635–1639 (2008)
R.M. Cornell, U. Schwertman, The Iron Oxides: properties, Reactions, Occurrences and Uses (VCH, New York, 1996), pp. 1–25
W. Wang, J.Y. Howe, B. Gu, J. Phys. Chem. C. 112, 25–9203 (2008)
R. Zboril, M. Mashlan, D. Petridis, Chem. Mater. 14, 969 (2002)
N. Pailhe, A. Wattiaux, M. Gaudon, A. Demourgues, J. Solid State Chem. 181, 2697 (2008)
A. Kay, I. Cesar, M. Gratzel, J. Am. Chem. Soc. 128, 15714 (2006)
I. Cesar, A. Kay, J.A.G. Martinez, M. Gratzel, J. Am. Chem. Soc. 128, 4582 (2006)
A. Duret, M. Gratzel, J. Phys. Chem. B 5 109, 17184 (2005)
C. Wu, P. Yin, X. Zhu, C. OuYang, Y. Xie, J. Phys. Chem. B. 110, 17806 (2006)
H.C. Yang, X.B. Mao, Y.J. Guo, D.W. Wang, G.L. Ge, R. Yang et al., CrystEngComm 12, 1842 (2010)
S. Wagloehner, D. Reichert, D.L. Sorzano, P. Balle, B. Geiger, S. Kureti, J. Catal. 260, 305 (2008)
X.L. Hu, J.C. Yu, Adv. Funct. Mater. 18, 880 (2008)
R. Zboril, M. Mashlan, D. Petridis, Chem. Mater. 14, 969 (2004)
L.H. Han, H. Liu, Y. Wei, Powder Technology. doi:10.1016/j.powtec.2010.10.008 (2010)
S. Akbar, S.K. Hasanain, N. Azmat, M. Nadeem, Condensed Matter 480, 1 (2004)
K. Hiroaki, K. Sridhar, J. Am. Ceram. Soc. 84, 2313 (2001)
M. Ocaña, M.P. Morales, C.J. Serna, J. Colloid Interface Sci. 171, 85 (2002)
L. Lu, L. Li, X. Wang, G. Li, J. Phys. Chem. B 109, 7151 (2005)
P. Ayyub, M. Multani, M. Barma, V.R. Palkar, R. Vijayaraghavan, J. Phys. C: Solid State Phys. 21, 229 (1988)
L.X. Chen, T. Liu, M. Thurnauer, R. Csencsits, T. Rajh, J. Phys. Chem. B 106, 8539 (2002)
V. Chernyshova, M.F. Hochella Jr, A.S. Madden, Phys.chem.chem.phys. 9, 1736 (2005)
M. Hanesch, Geophys. J. Int. 177, 941 (2009)
A. Zoppi, C. Lofrumento, E.M. Castellucci, Ph. Sciau, J. Raman Spectrosc. 39, 40 (2008)
I. Chourpa, L. Douziech-Eyrolles, L. Ngaboni-Okassa, J.F. Fouquenet, S. Cohen-Jonathan, M. Soucé, H. Marchais, P. Dubois, Analyst 130, 1395 (2005)
S.J. Oh, D.C. Cook, H.E. Townsend, Corrs. Sci. 41, 1687 (1999)
G. Grundmeier, M. Stratmann, Appl. Surf. Sci 141, 43 (1999)
J. Zuo, C. Xu, J. Raman Spectrosc 27, 921 (1997)
Acknowledgments
The authors acknowledge nanotechnology center, SRM University for XRD measurement. We thank Dr. R.V. Subba Rao and Dr. R.K. Dayal, Corrosion Science and Technology Division, IGCAR for MicroRaman measurements. We also thank Dr. A.K. Arora, Dr. B.V.R. Tata and Dr. C.S. Sundar, Materials Science Group, IGCAR for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh, R., Sohila, S., Muthamizhchelvan, C. et al. Synthesis and vibrational properties of hematite (α-Fe2O3) nanoparticles. J Mater Sci: Mater Electron 22, 1357–1360 (2011). https://doi.org/10.1007/s10854-011-0313-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-011-0313-1