Abstract
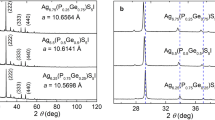
The crystal structure of Ag7(Si0.8Ge0.2)S5I, Ag7(Si0.6Ge0.4)S5I, Ag7(Si0.4Ge0.6)S5I and Ag7(Si0.2Ge0.8)S5I solid electrolytes, grown by vertical zone crystallization method (solution–melt technique), was studied using X-ray diffraction analysis by the Rietveld refinement method. The electrical conductivity of Ag7(Si1 − xGex)S5I solid solutions were measured by impedance spectroscopy technique in frequency range from 10 Hz to 2 × 106 Hz and temperature interval from 293 to 383 K. The compositional dependences of ionic part of total electrical conductivity and its activation energy were analyzed. The main features of the ion transport mechanism in Ag7(Si1 − xGex)S5I solid solutions caused by Si4+ ↔ Ge4+cationic substitution are explained via simple structural descriptors (SOF, dmax, ECoN). The spectral dependences of refractive index for Ag7(Si1 − xGex)S5I solid electrolytes were measured by the spectral ellipsometry technique. The spectrometric measurements of optical absorption edge in Ag7(Si1 − xGex)S5I solid solutions were carried out at room temperature. The compositional variation of energy pseudogap, Urbach energy and refractive indices in Ag7(Si1 − xGex)S5I solid solutions were studied.
Graphical abstract











Similar content being viewed by others
References
Gür TM (2018) Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ Sci 11:2696–2767. https://doi.org/10.1039/C8EE01419A
Abbas Q, Mirzaeian M, Hunt MRC, Hall P, Raza R (2020) Current state and future prospects for electrochemical energy storage and conversion systems. Energies 13(21):5847. https://doi.org/10.3390/en13215847
Grey CP, Hall DS (2020) Prospects for lithium-ion batteries and beyond—a 2030 vision. Nat Commun 11:6279. https://doi.org/10.1038/s41467-020-19991-4
Armand M, Axmann P, Bresser D, Copley M, Edström K, Ekberg C, Guyomard D, Lestriez B, Novák P, Petranikova M, Porcher W, Trabesinger S, Wohlfahrt-Mehrens M, Zhang H (2020) Lithium-ion batteries–current state of the art and anticipated developments. J Power Sour 479:228708. https://doi.org/10.1016/j.jpowsour.2020.228708
Lain MJ, Brandon J, Kendrick E (2019) Design strategies for high power vs high energy lithium ion cells. Batteries 5:64. https://doi.org/10.3390/batteries5040064
Chen Y, Kang Y, Zhao Y, Wang L, Liu J, Li Y, Liang Z, He X, Li X, Tavajohi N, Li B (2021) A review of lithium-ion battery safety concerns: the issues, strategies, and testing standards. J Energy Chem 59:83–99. https://doi.org/10.1016/j.jechem.2020.10.017
Quartarone E, Mustarelli P (2020) Review-emerging trends in the design of electrolytes for lithium and post-lithium batteries. J Electrochem Soc 167:050508. https://doi.org/10.1149/1945-7111/ab63c4
Bai X, Duan Y, Zhuang W, Yang R, Wang J (2020) Research progress in Li-argyrodite-based solid-state electrolytes. J Mater Chem A 8:25663–25686. https://doi.org/10.1039/D0TA08472G
Ohno S, Banik A, Dewald GF, Kraft MA, Krauskopf T, Minafra N, Till P, Weiss M, Zeier WG (2020) Materials design of ionic conductors for solid state batteries. Prog Energy 2:022001. https://doi.org/10.1088/2516-1083/ab73dd
Zhao Q, Stalin S, Zhao CZ, Archer LA (2020) Designing solid-state electrolytes for safe, energy-dense batteries. Nat Rev Mater 5:229–252. https://doi.org/10.1038/s41578-019-0165-5
Keen DA (2002) Disordering phenomena in superionic conductors. J Phys Condens Matter 14:R819–R857. https://doi.org/10.1088/0953-8984/14/32/201
Zheng F, Kotobuki M, Song S, Lai MO, Lu L (2018) Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sour 389:198–213. https://doi.org/10.1016/j.jpowsour.2018.04.022
Cao C, Li ZB, Wang XL, Zhao XB, Han WQ (2014) Recent advances in inorganic solid electrolytes for lithium batteries. Front Energy Res 2:25. https://doi.org/10.3389/fenrg.2014.00025
Zhou Q, Wang L, Li W, Zhao K, Liu M, Wu Q, Yang Y, He G, Parkin IP, Shearing PR, Brett DJL, Zhang J, Sun X (2021) Sodium superionic conductors (NASICONs) as cathode materials for sodium-ion batteries. Electrochem Energy Rev 4:793–823. https://doi.org/10.1007/s41918-021-00120-8
Song S, Duong HM, Korsunsky AM, Hu N, Lu L (2016) A Na(+) superionic conductor for room-temperature sodium batteries. Sci Rep 6:32330. https://doi.org/10.1038/srep32330
Studenyak IP, Luchynets MM, Izai VYu, Pogodin AI, Kokhan OP, Azhniuk YuM, Zahn DRT (2019) Structural and optical properties of (Cu6PS5Br)1–x(Cu7PS6)x mixed crystals. J Alloy Compd 782:586–591. https://doi.org/10.1016/j.jallcom.2018.12.214
Yamamoto O (2017) Solid state ionics: a Japan perspective. Sci Technol Adv Mater 18:504–527. https://doi.org/10.1080/14686996.2017.1328955
Yamada H, Bhattacharyya A, Maier J (2006) Extremely high silver ionic conductivity in composites of silver halide (AgBr, AgI) and mesoporous alumina. Adv Funct Mater 16:525–530. https://doi.org/10.1002/adfm.200500538
Yamamoto T, Maesato M, Hirao N, Kawaguchi SI, Kawaguchi S, Ohishi Y, Kubota Y, Kobayashi H, Kitagawa H (2017) The room-temperature superionic conductivity of silver iodide nanoparticles under pressure. J Am Chem Soc 139:1392–1395. https://doi.org/10.1021/jacs.6b11379
Zhang Z, Shao Y, Lotsch B, Hu YS, Li H, Janek J, Nazar LF, Nan CW, Maier J, Armand M, Chen L (2018) New horizons for inorganic solid state ion conductors. Energy Environ Sci 11:1945–1976. https://doi.org/10.1039/C8EE01053F
Zou Z, Li Y, Lu Z, Wang D, Cui Y, Guo B, Li Y, Liang X, Feng J, Li H, Nan CW, Armand M, Chen L, Xu K, Shi S (2020) Mobile ions in composite solids. Chem Rev 120:4169–4221. https://doi.org/10.1021/acs.chemrev.9b00760
Grady ZA, Wilkinson CJ, Randall CA, Mauro JC (2020) Emerging role of non-crystalline electrolytes in solid-state battery research. Front Energy Res 8:218. https://doi.org/10.3389/fenrg.2020.00218
Gao Z, Sun H, Fu L, Ye F, Zhang Y, Luo W, Huang Y (2018) Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv Mater 30:e1705702. https://doi.org/10.1002/adma.201705702
Wang Z, Wang D, Zou Z, Song T, Ni D, Li Z, Shao X, Yin W, Wang Y, Luo W, Wu M, Avdeev M, Xu B, Shi S, Ouyang C, Chen L (2020) Efficient potential-tuning strategy through p-type doping for designing cathodes with ultrahigh energy density. Nat Sci Rev 7:1768–1775. https://doi.org/10.1093/nsr/nwaa174
Chen Z, Zhang W, Yang Z (2020) A review on cathode materials for advanced lithium ion batteries: microstructure designs and performance regulations. Nanotechnology 31:012001. https://doi.org/10.1088/1361-6528/ab4447
Nourhan M, Nageh KA (2020) Recent advances in the design of cathode materials for Li-ion batteries. RSC Adv 10:21662–21685. https://doi.org/10.1039/D0RA03314F
Zhang L, He B, Zhao Q, Zou Z, Chi S, Mi P, Ye A, Li Y, Wang D, Avdeev M, Adams S, Shi S (2020) A database of ionic transport characteristics for over 29 000 inorganic compounds. Adv Funct Mater 30:2003087. https://doi.org/10.1002/adfm.202003087
Chen HM, Maohua C, Adams S (2015) Stability and ionic mobility in argyrodite-related lithium-ion solid electrolytes. Phys Chem Chem Phys 17:16494–16506. https://doi.org/10.1039/C5CP01841B
Zhao Q, Zhang L, He B, Ye A, Avdeev M, Chen L, Shi S (2021) Identifying descriptors for Li+ conduction in cubic Li-argyrodites via hierarchically encoding crystal structure and inferring causality. Energy Storage Materials 40:386–393. https://doi.org/10.1016/j.ensm.2021.05.033
Zhao Q, Avdeev M, Chen L, Shi S (2021) Machine learning prediction of activation energy in cubic Li-argyrodites with hierarchically encoding crystal structure-based (HECS) descriptors. Sci Bull 66:1401–1408. https://doi.org/10.1016/j.scib.2021.04.029
He B, Chi S, Ye A, Mi P, Zhang L, Pu B, Zou Z, Ran Y, Zhao Q, Wang D, Zhang W, Zhao J, Adams S, Avdeev M, Shi S (2020) High-throughput screening platform for solid electrolytes combining hierarchical ion-transport prediction algorithms. Sci Data 7:151. https://doi.org/10.1038/s41597-020-0474-y
He B, Ye A, Chi S, Mi P, Ran Y, Zhang L, Zou X, Pu B, Zhao ZZ, Wang D, Zhang W, Zhao J, Avdeev M, Shi S (2020) CAVD, towards better characterization of void space for ionic transport analysis. Sci Data 7:153. https://doi.org/10.1038/s41597-020-0491-x
He B, Mi P, Ye A, Chi S, Jiao Y, Zhang L, Pu B, Zou Z, Zhang W, Avdeev M, Adams S, Zhao J, Shi S (2021) A highly efficient and informative method to identify ion transport networks in fast ion conductors. Acta Mater 203:116490. https://doi.org/10.1016/j.actamat.2020.116490
Pan L, Zhang L, Ye A, Chi S, Zou Z, He B, Chen L, Zhao Q, Wang D, Shi S (2019) Revisiting the ionic diffusion mechanism in Li3PS4 via the joint usage of geometrical analysis and bond valence method. J Materiomics 5:688–695. https://doi.org/10.1016/j.jmat.2019.04.010
Kuhs WF, Nitsche R, Scheunemann K (1979) The argyrodites—a new family of tetrahedrally close-packed structures. Mat Res Bull 14:241–248. https://doi.org/10.1016/0025-5408(79)90125-9
Nilges T, Pfitzner A (2005) A structural differentiation of quaternary copper argyrodites: structure—property relations of high temperature ion conductors. Z Kristallogr 220:281–294. https://doi.org/10.1524/zkri.220.2.281.59142
Deiseroth HJ, Maier J, Weichert K, Nickel V, Kong ST, Reiner C (2011) Li7PS6 and Li6PS5X (X: Cl, Br, I): possible three-dimensional diffusion pathways for lithium ions and temperature dependence of the ionic conductivity by impedance measurements. Z Anorg Allg Chem 637:1287–1294. https://doi.org/10.1002/zaac.201100158
Beeken RB, Garbe JJ, Gillis JM, Petersen NR, Podoll BW, Stoneman MR (2005) Electrical conductivities of the Ag6PS5X and the Cu6PSe5X (X=Br, I) argyrodites. J Phys Chem Solids 66:882–886. https://doi.org/10.1016/j.jpcs.2004.10.010
Hanghofer I, Brinek M, Eisbacher SL, Bitschnau B, Volck M, Hennige V, Hanzu I, Rettenwander D, Wilkening HMR (2019) Substitutional disorder: structure and ion dynamics of the argyrodites Li6PS5Cl, Li6PS5Br and Li6PS5I. Phys Chem Chem Phys 21:8489–8507. https://doi.org/10.1039/C9CP00664H
Laqibi M, Cros B, Peytavin S, Ribes M (1987) New silver superionic conductors Ag7XY5Z (X = Si, Ge, Sn; Y = S, Se; Z = Cl, Br, I)-synthesis and electrical studies. Solid State Ionics 23:21–26. https://doi.org/10.1016/0167-2738(87)90077-4
Gaudin E, Petricek V, Boucher F, Taulelle F, Evain M (2000) Structures and phase transitions of the A7PSe6 (A = Ag, Cu) argyrodite-type ionic conductors. III. alpha-Cu7PSe6. Acta Crystallogr B 56:972–979. https://doi.org/10.1107/s0108768100010260
Kraft MA, Ohno S, Zinkevich T, Koerver R, Culver SP, Fuchs T, Senyshyn A, Indris S, Morgan BJ, Zeier WG (2018) Inducing high ionic conductivity in the lithium superionic argyrodites Li6+xP1−xGexS5I for all-solid-state batteries. J Am Chem Soc 140:16330–16339. https://doi.org/10.1021/jacs.8b10282
Tomm Y, Schorr S, Fiechter S (2008) Crystal growth of argyrodite-type phases Cu8–xGeS6-xIx and Cu8–xGeSe6–xIx (0≤x≤0.8). J Cryst Growth 210:2215–2221. https://doi.org/10.1016/j.jcrysgro.2007.11.184
Pogodin AI, Filep MJ, Malakhovska TO, Sabov MYu, Sidey VI, Kokhan OP, Studenyak IP (2019) The copper argyrodites Cu7–nPS6–nBrn: crystal growth, structures and ionic conductivity. Solid State Ion 341:115023. https://doi.org/10.1016/j.ssi.2019.115023
Studenyak IP, Pogodin AI, Kokhan OP, Kavaliukė V, Šalkus T, Kežionis A, Orliukas AF (2019) Crystal growth, structural and electrical properties of (Cu1−xAgx)7GeS5I superionic solid solutions. Solid State Ion 329:119–123. https://doi.org/10.1016/j.ssi.2018.11.020
Studenyak IP, Pogodin AI, Studenyak VI, Izai VYu, Filep MJ, Kokhan OP, Kranjčec M, Kúš P (2020) Electrical properties of copper- and silver-containing superionic (Cu1−xAgx)7SiS5I mixed crystals with argyrodite structure. Solid State Ion 345:115183. https://doi.org/10.1016/j.ssi.2019.115183
Studenyak IP, Pogodin AI, Studenyak VI, Filep MJ, Kokhan OP, Kús P, Azhniuk YM, Zahn DRT (2021) Structure, electrical conductivity, and Raman spectra of (Cu1−xAgx)7GeS5I and (Cu1−xAgx)7GeSe5I mixed crystals. Mat Res Bull 135:111116. https://doi.org/10.1016/j.materresbull.2020.111116
Studenyak IP, Pogodin AI, Filep MJ, Symkanych OI, Babuka TY, Kokhan OP, Kúš P (2021) Influence of heterovalent cationic substitution on electrical properties of Ag6+x(P1−xGex)S5I solid solutions. J Alloy Compd 873:159784. https://doi.org/10.1016/j.jallcom.2021.159784
Fan Y, Wang G, Wang R, Zhang B, Shen X, Jiang P, Zhang X, Gu H, Lu X, Zhou X (2020) Enhanced thermoelectric properties of p-type argyrodites Cu8GeS6 through Cu vacancy. J Alloy Compd 822:53665. https://doi.org/10.1016/j.jallcom.2020.153665
Studenyak IP, Pogodin AI, Luchynets MM, Filep MY, Kohutych AA, Malakhovska TO, Kokhan OP, Sabov MYu, Kúš P (2021) Influence of heterovalent substitution on structural, electrical and thermoelectric properties of Cu7−xPS6−xBrx solid solutions. J Phys Chem Solids 150:109855. https://doi.org/10.1016/j.jpcs.2020.109855
Samulionis V, Banys J, Vysochanskii Y, Studenyak I (2006) Investigation of ultrasonic and acoustoelectric properties of ferroelectric-semiconductor crystals. Ferroelectrics 336:29–38. https://doi.org/10.1080/00150190600695255
Studenyak IP, Kranjec M, GyS K, Desnica-Frankovic ID, Panko VV, Slivka VYu (2001) The excitonic processes and Urbach rule in Cu6P(S1-xSex)5I crystals in the sulfur-rich region. Mat Res Bull 36:123–135. https://doi.org/10.1016/S0025-5408(01)00508-6
Kranjčec M, Studenyak IP, Kurik MV (2006) Urbach rule and disordering processes in Cu6P(S1−xSex)5Br 1−yIy superionic conductors. J Phys Chem Solids 67:807–817. https://doi.org/10.1016/j.jpcs.2005.10.184
Studenyak IP, Izai VYu, Studenyak VI, Pogodin AI, Filep MY, Kokhan OP, Kúš GB, P, (2018) Interrelations between structural and optical properties of (Cu1−xAgx)7GeS5I mixed crystals. Ukr J Phys Opt 19:237–243. https://doi.org/10.3116/16091833/19/4/237/2018
Studenyak IP, Pogodin AI, Shender IA, Filep MJ, Kokhan OP, Kopčanský P (2021) Electrical properties of cation-substituted Ag7(Si1−xGex)S5I single crystals. Semicond Phys Quantum Electron Optoelectron 24(3):241–247. https://doi.org/10.15407/spqeo24.03.241-247
McCusker LB, Von Dreele RB, Cox DE, Louër D, Scardi P (1999) Rietveld refinement guidelines. J Appl Crystallogr 32:36–50. https://doi.org/10.1107/S0021889898009856
Altomare A, Cuocci C, Giacovazzo C, Moliterni A, Rizzi R, Corriero N, Falcicchio A (2013) EXPO2013: a kit of tools for phasing crystal structures from powder data. J Appl Crystallogr 46:1231–1235. https://doi.org/10.1107/S0021889813013113
Momma K, Izumi F (2011) VESTA 3 for three-dimen-sional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276. https://doi.org/10.1107/S0021889811038970
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I, Dal Corso A, de Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen AP, Smogunov A, Umari P, Wentzcovitch RM (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21:395502. https://doi.org/10.1088/0953-8984/21/39/395502
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133. https://doi.org/10.1103/PhysRev.140.A1133
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
Vanderbilt D (1990) Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B 41:7892–7895. https://doi.org/10.1103/PhysRevB.41.7892
Monkhorst HJ, Pack JD (1976) Special points for brillouin-zone integrations. Phys Rev B 13:5188–5192. https://doi.org/10.1103/PhysRevB.13.5188
Babuka T, Glukhov K, Kohutych A, Vysochanskii Y, Makowska-Janusik M (2020) Nature of thermoelectric properties formation in defected Sn2P2S6 chalcogenide crystals. CrystEngComm 22:2336–2349. https://doi.org/10.1039/C9CE02017A
Huggins RA (2002) Simple method to determine electronic and ionic components of the conductivity in mixed conductors a review. Ionics 8:300–313. https://doi.org/10.1007/BF02376083
Hoppe R (1979) Effective coordination numbers (ECoN) and mean fictive ionic radii (MEFIR). Z Kristallogr 150:23–52. https://doi.org/10.1524/zkri.1979.150.14.23
Giacovazzo C, Monaco HL, Artioli G, Viterbo D, Milanesio M, Gilli G, Gilli P, Zanotti G, Ferraris G, Catti M (2011) Fundamentals of crystallography, 3rd edn. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780199573653.001.0001
Tinoco T, Quintero M, Rinkon C (1991) Variation of the energy gap with composition in AIBIIIC2VI chalcopyrite-structure alloys. Phys Rev B 44(4):1613–1615. https://doi.org/10.1103/PhysRevB.44.1613
Kurik MV (1971) Urbach rule. Physica Status Solidi (a) 8:9–45. https://doi.org/10.1002/pssa.2210080102
Skumanich A, Frova A, Amer NM (1985) Urbach tail and gap states in hydrogenated a-SiC and a-SiGe alloys. Solid State Commun 54(7):597–601. https://doi.org/10.1016/0038-1098(85)90086-9
Wemple SH, Di Domenico M (1971) Behaviour of the dielectric constant in covalent and ionic materials. Phys Rev B 3:1338–1352. https://doi.org/10.1103/PhysRevB.3.1338
Tanaka K (1980) Optical properties and photoinduced changes in amorphous As-S films. Thin Solid Films 66:271–279. https://doi.org/10.1016/0040-6090(80)90381-8
Tubbs MS (1970) A spectroscopic interpretation of crystalline iconicity. Phys Stat Sol (b) 41:K61–K64. https://doi.org/10.1002/pssb.19700410164
Acknowledgements
This research was supported by project “Flexible Magnetic Filaments: Properties and Applications” (FMF; M-ERA.NET 2). Research was also supported by the project “Modified (nano) textile materials for health technologies” (No. ITMS 313011T548, Structural Funds of EU, Ministry of Education, Slovakia) and by the Slovak Research and Development Agency under the Contract No. APVV-15-0453.
Author information
Authors and Affiliations
Contributions
AP: Visualization, Investigation, Writing- original draft. IS: Conceptualization, Supervision. IS: Visualization, Investigation. MP: Visualization, Investigation, Writing- original draft. MF: Visualization, Investigation, Writing- original draft. TM: Methodology, Validation. OK: Conceptualization, Methodology. PK: Writing- review & editing. TB: Software, Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Kyle Brinkman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pogodin, A.I., Studenyak, I.P., Shender, I.A. et al. Crystal structure, ion transport and optical properties of new high-conductivity Ag7(Si1 − xGex)S5I solid solutions. J Mater Sci 57, 6706–6722 (2022). https://doi.org/10.1007/s10853-022-07059-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07059-1