Abstract
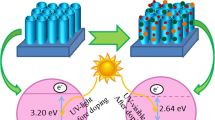
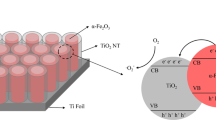
In this study, TiO2 nanotube (TiO2NT) arrays were successfully synthesized via two-step anodization in organic electrolyte solutions composed of ethylene glycol, 0.5 wt% NH4F, and 3.0 wt% H2O. The effect of aged electrolytes on the morphological and photocatalytic properties of the obtained TiO2NTs was investigated. Field-emission scanning electron microscopy, X-ray diffractometry, UV–Vis absorption spectrometry, and photoluminescence measurements were also conducted to evaluate the physicochemical properties of the TiO2NTs synthesized with electrolytes of various ages. The photocatalytic activity of the TiO2NTs was characterized by photocurrent measurements and phenol degradation assay. The results confirmed the strong dependence of the morphology of the NTs on the aged electrolyte. Anodization of Ti foil with aged electrolytes led to not only reductions in morphological defects but also enhancement of the order of the TiO2NTs. The longer the aging time, the thicker the NT wall and the shorter the tube length obtained. Prolonged aging of the electrolyte resulted in the formation of TiO2NTs with a nanoporous surface, which could be attributed to the deposition of Ti(OH)4. The results collectively indicate that improvements in the photocatalytic property of the TiO2NT samples could be obtained when synthesis is conducted with aged electrolytes. The aging process of the electrolytes was clearly demonstrated to promote the structural integrity and photocatalytic ability of the obtained TiO2NTs.
Graphical abstract











Similar content being viewed by others
References
Zwilling V, Darque-Ceretti E, Boutry-Forveille A, David D, Perrin MY, Aucouturier M (1999) Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf Interface Anal 27:629–637. https://doi.org/10.1002/(sici)1096-9918(199907)27:7%3c629::aid-sia551%3e3.0.co;2-0
Shankar K, Basham JI, Allam NK et al (2009) Recent advances in the use of TiO2 nanotube and nanowire arrays for oxidative photoelectrochemistry. J Phys Chem C 113:6327–6359. https://doi.org/10.1021/jp809385x
Roy P, Berger S, Schmuki P (2011) TiO2 nanotubes: synthesis and applications. Angew Chem Int Ed 50:2904–2939. https://doi.org/10.1002/anie.201001374
Lee D, Kim HB, Yu S, Kim HJ, Lee WI (2014) Jang DJ (2014) Facile fabrication of anatase TiO2 nanotube arrays having high photocatalytic and photovoltaic performances by anodization of titanium in mixed viscous solvents. J Mater Sci 49:3414–3422
Hu MZ, Lai P, Bhuiyan MS, Tsouris C, Gu BH, Paranthaman MP, Gabitto J, Harrison L (2009) Synthesis and characterization of anodized titanium-oxide nanotube arrays. J Mater Sci 44:2820–2827
Raja KS, Gandhi T, Misra M (2007) Effect of water content of ethylene glycol as electrolyte for synthesis of ordered titania nanotubes. Electrochem commun 9:1069–1076. https://doi.org/10.1016/j.elecom.2006.12.024
Gong D, Grimes CA, Varghese OK et al (2001) Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res 16:3331–3334. https://doi.org/10.1557/jmr.2001.0457
Varghese OK, Gong DW, Paulose M, Grimes CA, Dickey EC (2003) Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. J Mater Res 18:156–165. https://doi.org/10.1557/jmr.2003.0022
Macak JM, Tsuchiya H, Schmuki P (2005) High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew Chem Int Ed 44:2100–2102. https://doi.org/10.1002/anie.200462459
Cai QY, Paulose M, Varghese OK, Grimes CA (2005) The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. J Mater Res 20:230–236. https://doi.org/10.1557/jmr.2005.0020
Indira K, Ningshen S, Mudali K, Rajendran N (2011) Effect of anodization temperature on the surface morphology of anodized titanium. In: Jayakumar S, Kannan MD, Balasundaraprabhu R, Prassana S (eds) Thin films and nanomaterials. Macmillan Publishers, New York, pp 63–66
Han SC, Doh JM, Yoon JK et al (2009) Highly ordered self-organized TiO2 nanotube arrays prepared by a multi-step anodic oxidation process. Met Mater Int 15:493. https://doi.org/10.1007/s12540-009-0493-x
Macak JM, Tsuchiya H, Taveira L, Aldabergerova S, Schmuki P (2005) Smooth anodic TiO2 nanotubes. Angew Chem Int Ed 44:7463–7465. https://doi.org/10.1002/anie.200502781
Bauer S, Kleber S, Schmuki P (2006) Tailoring the geometry in H3PO4/HF electrolytes. Electrochem commun 8:1321–1325. https://doi.org/10.1016/j.elecom.2006.05.030
Yang Y, Wang X, Li L (2008) Synthesis and growth mechanism of graded TiO2 nanotube arrays by two-step anodization. Mater Sci Eng B 149:58–62
Gong J, Lai Y, Lin C (2010) Electrochemically multi-anodized TiO2 nanotube arrays for enhancing hydrogen generation by photo-electrocatalytic water splitting. Electrochim Acta 55:4776–4782
Albu SP, Ghicov A, Macak JM, Schmuki P (2007) 250 µm long anodic TiO2 nanotubes with hexagonal self-ordering. Phys Status Solidi Rapid Res Lett 1:R65–R67. https://doi.org/10.1002/pssr.200600069
Macak JM, Tsuchiya H, Ghicov A et al (2007) TiO2 nanotubes: self-organized electrochemical formation, properties and applications. Curr Opin Solid State Mater Sci 11:3–18. https://doi.org/10.1016/j.cossms.2007.08.004
Ghicov A, Schmuki P (2009) Self-ordering electrochemistry: a review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem Commun. https://doi.org/10.1039/b822726h
Tian T, Xiao XF, Liu RF, She HD, Hu XF (2007) Study on titania nanotube arrays prepared by titanium anodization in NH4F/H2SO4 solution. J Mater Sci 42:5539–5543
Elsanousi A, Zhang J, Fadlalla HMH, Zhang F, Wang H, Ding X, Huang Z, Tang C (2008) Self-organized TiO2 nanotubes with controlled dimensions by anodic oxidation. J Mater Sci 43:7219–7224
Ruan CM, Paulose M, Varghese OK, Mor GK, Grimes CA (2005) Fabrication of highly ordered TiO2 nanotube arrays using an organic electrolyte. J Phys Chem B 109:15754–15759. https://doi.org/10.1021/jp052736u
Yang DJ, Kim HG, Cho SJ, Choi WY (2008) Thickness-conversion ratio from titanium to TiO2 nanotube fabricated by anodization method. Mater Lett 62:775–779. https://doi.org/10.1016/j.matlet.2007.06.058
Paulose M, Shankar K, Yoriya S et al (2006) Anodic growth of highly ordered TiO2 nanotube arrays to 134 µm in length. J Phys Chem B 110:16179–16184. https://doi.org/10.1021/jp064020k
Li SQ, Zhang GM, Guo DZ, Yu LG, Zhang W (2009) Anodization fabrication of highly ordered TiO2 nanotubes. J Phys Chem C 113:12759–12765. https://doi.org/10.1021/jp903037f
Sopha H, Hromadko L, Nechvilova K, Macak JM (2015) Effect of electrolyte age and potential changes on the morphology of TiO2 nanotubes. J Electroanal Chem 759:122–128. https://doi.org/10.1016/j.jelechem.2015.11.002
Suhadolnik L, Marinko Z, Ponikvar-Svet M, Tavcar G, Kovac J, Ceh M (2020) Influence of anodization-electrolyte aging on the photocatalytic activity of TiO2 nanotube arrays. J Phys Chem C 124:4073–4080. https://doi.org/10.1021/acs.jpcc.9b09522
Peng ZX, Ni JH (2019) Surface properties and bioactivity of TiO2 nanotube array prepared by two-step anodic oxidation for biomedical applications. Royal Soc Open Sci. https://doi.org/10.1098/rsos.181948
Jarosz M, Pawlik A, Kapusta-Kolodziej J, Jaskula M, Sulka GD (2014) Effect of the previous usage of electrolyte on growth of anodic titanium dioxide (ATO) in a glycerol-based electrolyte. Electrochim Acta 136:412–421
Balaur E, Macak JM, Tsuchiya H, Schmuki P (2005) Wetting behaviour of layers of TiO2 nanotubes with different diameters. J Mater Chem 15:4488–4491. https://doi.org/10.1039/b509672c
Josepha ST, Davidb MC, Sagayaraja P (2014) The role of electrolyte pH in enhancing the surface morphology of TiO2 nanotube arrays grown on Ti substrate. Int J Sci Eng Res 5(3):85–91
Macak JM, Sirotna K, Schmuki P (2005) Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochim Acta 50:3679–3684
Yoo J, Lee K, Tighineanu A, Schmuki P (2013) Highly ordered TiO2 nanotube-stumps with memristive response. Electrochem Commun 34:177–180. https://doi.org/10.1016/j.elecom.2013.05.038
Mahajan VK, Misra M, Raja KS, Mohapatra SK (2008) Self-organized TiO2 nanotubular arrays for photoelectrochemical hydrogen generation: effect of crystallization and defect structures. J Phys D 41:125307–125316. https://doi.org/10.1088/0022-3727/41/12/125307
Tang H, Berger H, Schmid PE, Lévy F, Burri G (1993) Photoluminescence in TiO2 anatase single crystals. Solid State Commun 87:847–850
Lei Y, Zhang LD, Meng GW, Li GH, Zhang XY, Liang CH, Chen W, Wang SX (2001) Preparation and photoluminescence of highly ordered TiO2 nanowire arrays. Appl Phys Lett 78:1125–1127
Serpone N, Lawless D, Khairutdinov RJ (1995) size effects on the photophysical properties of colloidal anatase TiO2 particles: size quantization or direct transitions in this indirect semiconductor. Phys Chem 99:16646–16654
Saraf LV, Patil SI, Ogale SB, Sainkar SR, Kshirsager ST (1998) Synthesis of nanophase TiO2 by ion beam sputtering and cold condensation technique. Int J Mod Phys B 12:2635–2647
Toyozawa Y (1970) Phonon structures in the spectra of solids. J Lumin 1–2:732–774
Liu B, Peng L (2013) Facile formation of mixed phase porous TiO2 nanotubes and enhanced visible-light photocatalytic activity. J Alloys Compd 571:145–152
Li X, Gao C, Wang J et al (2012) TiO2 films with rich bulk oxygen vacancies prepared by electrospinning for dye-sensitized solar cells. J Power Sour 214:244–250
Vijayan PP, Thomas M, George KC (2012) Effect of calcinations on electrical properties of TiO2 nanotubes. J Appl Phys 112:104308–104316
Acknowledgements
We thank the financial support provided by the Ministry of Science and Technology Taiwan (MOST 109-2221-E-029-006) and Ministry of Education for the New Southbound Talent Development Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luan, N.H., Chang, CF. Investigation of the aging effect of electrolyte on the morphology and photocatalytic properties of TiO2NTs synthesized using the anodization route. J Mater Sci 56, 19106–19118 (2021). https://doi.org/10.1007/s10853-021-06433-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06433-9