Abstract
Featuring high levels of achievable oxygen non-stoichiometry δ, Ce1−xZrxO2−δ solid solutions (CZO) are crucial for application as oxygen storage materials in, for example, automotive three-way catalytic converters (TWC). The use of CZO in form of films combined with simple manufacturing methods is beneficial in view of device miniaturization and reducing of TWC manufacturing costs. In this study, a comparative microstructural and electrochemical characterization of film and conventional bulk CZO is performed using X-ray diffractometry, scanning electron microscopy, and impedance spectroscopy. The films were composed of grains with dimensions of 100 nm or less, and the bulk samples had about 1 µm large grains. The electrical behavior of nanostructured films and coarse-grained bulk CZO (x > 0) was qualitatively similar at high temperatures and under reducing atmospheres. This is explained by dominating effect of Zr addition, which masks microstructural effects on electrical conductivity, enhances the reducibility, and favors strongly electronic conductivity of CZO at temperatures even 200 K lower than those for pure ceria. The nanostructured CeO2 films had much higher electrical conductivity with different trends in dependence on temperature and reducing atmospheres than their bulk counterparts. For the latter, the conductivity was dominantly electronic, and microstructural effects were significant at T < 700 °C. Nanostructural peculiarities of CeO2 films are assumed to induce their more pronounced ionic conduction at medium oxygen partial pressures and relatively low temperatures. The defect interactions in bulk and film CZO under reducing conditions are discussed in the framework of conventional defect models for ceria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ceria/zirconia mixed oxides Ce1–xZrxO2–δ (CZO) are crucial components of state-of-the-art technologies to minimize hazardous emissions of combustion engines in the environment. The most known devices to rely on CZO are the three-way catalytic converters (TWC) used for after treatment of the exhausts of most gasoline-powered vehicles [1,2,3,4,5,6]. The interest in CZO is motivated by its outstanding catalytic properties due to its much lower reduction enthalpies and higher levels of attainable oxygen non-stoichiometry δ and, hence, its superior oxygen storage or release capacity, in contrast to pure CeO2−δ [4,5,6,7,8,9,10,11,12,13,14,15,16]. The development of oxygen non-stoichiometries (facilitated by high temperatures and decreasing oxygen partial pressure, pO2) in ceria-based compounds is caused by the loss of oxygen and related formation of positively charged oxygen vacancies that are compensated by conduction electrons (reduced Ce). They enhance the electrical conductivity σ [14,15,16,17], which, thus, becomes an indirect measure of the reduction degree. In nominally undoped CZO, the favorable effect on the conductivity is assigned to the substitution of Zr for Ce. It exerts strain on the crystal lattice, thus, favoring an extensive formation of oxygen vacancies, which is associated with structural relaxation through reduction of Ce4+ to Ce3+ ions. This boosts the electronic transport at considerably higher pO2 and lower reduction temperatures than for pure CeO2−δ [4, 7, 10,11,12,13]. Despite the obvious influence of Zr addition, the reduction kinetics also depends upon structural peculiarities of the catalyst materials, e.g., on extended surfaces and on grain boundaries (GB) in thin films or nanostructured bulk ceramics [4, 7, 10,11,12,, 18,19,20,21,22,23,24,25,26]. In these low-dimensional CZO morphologies, even a further decrease in the reduction enthalpy and a strong increase in the electrical conductivity over that of bulk materials with coarse grains can be expected [24,25,26,27,28]. This implies larger non-stoichiometries for a given set of temperatures and pO2. From an application point of view, understanding the relations between microstructure and reducibility combined with utilization of simple fabrication methods would allow for the development of Ce1−xZrxO2–δ films with superior catalytic properties, paving the way toward less costly high-performance miniaturized TWCs.
Previously, we reported on the high-temperature electrical conductivity (σ) and non-stoichiometry (δ) of Ce1−xZrxO2−δ films in oxygen-deficient atmosphere [29], where DC measurements and impedance spectroscopy were applied for determining δ, in conjunction with a resonant nanobalance method. A correlation between the conductivity σ and non-stoichiometry δ of CZO films in terms of slopes derived from log–log representations of their pO2-dependences at 800–900 °C was evident. However, at lower temperatures (600–700 °C), the conductivity of CZO films, especially of pure CeO2−δ, barely correlated with the non-stoichiometry parameter δ. The question arose, whether the observed phenomena were specific to the fact that studied Ce1−xZrxO2−δ were films. To address this question, the present study aims to compare the electrical conductivities of bulk ceramics and films of Ce1−xZrxO2−δ with 0 ≤ x ≤ 0.67, basing on the fundamental prerequisite that they were prepared from the same starting materials to exclude different impurity levels. This should enable us to figure out whether possible differences
-
(1)
are specifically related to the dimensions and microstructures of films or
-
(2)
whether the electrochemical behavior of bulk and film Ce1−xZrxO2−δ could be equally described in the framework of conventional bulk defect chemical models for ceria derivatives.
The starting point is the microstructural characterization of both types of CZO samples. Subsequently, their electrical properties are measured and analyzed by impedance spectroscopy in two- and four-electrode configurations.
Materials and methods
Bulk ceramic specimens of CeO2–ZrO2 solid solutions (Ce1−xZrxO2−δ; CZO; 0 ≤ x ≤ 0.67) were fabricated by solid-state reactions with calcination and sintering at 1650 °C as in details reported in our previous work [29]. The resulting pellets served as targets for pulsed laser deposition (PLD) of CZO films onto sapphire substrates (Al2O3) using a 248 nm excimer KrF laser (COMPex, Coherent, Inc., Santa Clara, USA) operated at pulse rate of 10 Hz with 200 mJ pulse energy. The depositions were conducted in an evacuated chamber (5 × 10–4−10–3 Pa during sputtering) with no intentional heating of the substrates. After deposition, the films were heat-treated in air at 900 °C for 1 h in order to restore the oxygen stoichiometry [29].
The Ce1−xZrxO2−δ pellets and films were characterized by X-ray diffraction (XRD) in parallel beam (2θ–ω) geometry enabled by Göbel mirror within 2θ = (25−100)° with 0.02° resolution and 1300 s integration time per step using the Bruker D8 ADVANCE X-ray diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) with a Cu-Kα1 + Cu-Kα2 radiation (λ = 1.5419 Å). The pre-calibrated instrumental line broadening profile of the diffractometer was available from the device manufacturer, which enabled estimation of crystallites sizes by Scherrer’s equation [30]. A whole pattern fitting combined with a Rietveld refinement (WPF) [31, 32] was implemented for further evaluation of crystallographic properties of CZO. The microstructural and elemental analysis of Ce1−xZrxO2−δ was performed with scanning electron microscope and energy-dispersive X-ray spectroscopy (SEM/EDS, CamScan 44, CamScan Electron Optics Ltd., Waterbeach, UK) and, additionally, with a high-resolution SEM (Omicron NanoSAM, Scienta Omicron AB, Uppsala, Sweden). With a confidence level of 95.4%, the uncertainties of the determined elemental fractions were about 1.5% for Ce and Zr and 3.2% for oxygen. The Ce1−xZrxO2−δ bulk samples and PLD targets also contained non-intentional impurities of several 100 ppm [29], which may cause the formation of extrinsic oxygen vacancies (see sec. 3.3).
For electrical conductivity measurements, the bulk 1.5–2-mm-thick CZO pellets were cut into about 8 mm-long and 2.1–3.5 mm-wide bars. The PLD Ce1−xZrxO2−δ films had a size of 8 × 8 mm2 and thicknesses of about 2 µm (x = 0, 0.2 and 0.33) and 4–5 µm (x = 0.5, 0.67). The electrical conductivity of bulk and film CZO was analyzed in the four- (IS4) and two-contact (IS2) configuration, respectively, by impedance spectroscopy (IS) using the Solartron 1260 impedance gain-phase analyzer assisted by the high-impedance measuring bridge Solartron 1296 (Solartron Analytical, Leicester, UK). Two ring Pt electrodes were bound at the edges of the bulk bars (current leads) and another two ring electrodes—at 4–6 mm from each other in the middle of the bars (voltage leads). For films, two stripe electrodes were applied 2–4 mm away from each other by screen printing of a Pt paste and firing at 900 °C for 1 h in air. The uncertainties of geometric parameters for bulk and film samples are typically 1.5%, and the accuracy of average thicknesses of films is within error margins of 3.2–11.3%. The studied specimens are depicted in Fig. 1. The IS4 data were taken in a frequency range of 1–107 Hz with a rms AC voltage amplitude of 20 mV and the IS2—in the range of 10–106 Hz and a rms AC voltage of 50 mV. From the differences in both spectra, one may identify electrode effects [33] and exclude them from further interpretation of true electrical conductivity of CZO.
Additionally, the DC electrical conductivity of bulk and film CZO was determined in two-contact configuration (also applied for IS2 of bulks) by a Keithley DVM 2700 digital multimeter (Keithley Instruments, Solon, OH, USA). The conductivity was measured under isothermal conditions at 600, 700, 800, and 900 °C in a gastight furnace with a temperature control enabled by the Type S thermocouple located in the vicinity to the measured samples and by a Pt100 thermoresistor compensating the temperature drifts at the cold end of the thermocouple. To establish reducing atmosphere in the chamber, i.e., oxygen partial pressures pO2 between 10–26 and 0.2 bar, the oxygen ion pump technique was applied [34]. Small O2 amounts were admixed to the flow (20 mL/min) of a reducing gas mixture (99.5% Ar–0.5% H2). The uncertainty of evaluated electrical conductivities was in the range of 2–4% for bulk and 3–6% for film specimens. Since the sample dimensions do not contribute in the determination of slopes in σ(T) and σ(pO2) dependences of electrical conductivity, the uncertainties of these coefficients were lower and amounted to about 3%.
Results and discussion
In order to elucidate the possible differences in the temperature- and pO2-dependent behavior of film and bulk ceramic ceria/zirconia mixed oxides, first a comparative study of their microstructural peculiarities is performed. Then, the analysis of temperature dependences of their electrical conductivity in air and in reducing atmosphere using the impedance spectroscopy in 2- and 4-electrode configurations is conducted.
Microstructural and chemical characterization of Ce1−xZrxO2−δ
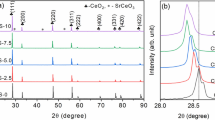
The starting point is the microstructural characterization of CZO by XRD and SEM/EDS. In Fig. 2, typical XRD patterns for bulk samples and PLD films are compared. Table 1 provides the relevant characteristics of Ce1−xZrxO2−δ, extracted by the WPF. The ceria-rich compounds (x = 0, 0.2 and 0.33) crystallized in face-centered cubic phase of the CaF2-type (Fm \({\overline{\text{3}}}\) m) [35], and ZrO2-rich materials (x = 0.5, 0.67) crystallized in metastable tetragonal P42/nmc phase [36]. The CZO films were found to fairly reproduce the crystalline structure of their bulk counterparts. However, we note a preferred orientation of the cubic CZO on sapphire, which is evidenced by the suppression of reflections that correspond to planes parallel to or inclined toward the a-axis (e.g., the (200) and (311) reflections in Fig. 2). Similar results for CeO2-based layers on Al2O3 substrates were reported in [23, 37].
The determined lattice parameters are consistent with those reported in the literature [7, 23, 35, 36] and, as expected, decrease linearly with increasing Zr content in cubic samples (Fig. 3, Table 1). The average grain sizes rc, both in bulk and film samples, show a trend to decrease with increasing Zr content (Table 1), but in PLD layers on sapphire, the grains were markedly smaller by a factor of about 20 (up to 40 in case of Ce0.8Zr0.2O2−δ). The above trend is at least for x = 0 and 0.2 consistent with the literature [19].
The chemical compositions of PLD and bulk CZO are also similar (Table 2). The PLD layers on sapphire are composed of large flat and homogeneous planar “islands” with lateral dimensions exceeding several tens of micrometers (Fig. 4b, d). A closer look into the interior of large planar features (inset in Fig. 4b) showed an array of regularly dispersed and densely packed grains with dimensions less than 200 nm. This agrees well with the grain size as determined by XRD. We note also the irregularly shaped relatively large particles randomly dispersed over the surface of films, whose surface density increased with ZrO2 fraction in the material, as previously reported in [29]. Within the measurement error, the EDS analysis of the particles revealed the same composition as the “islands”.
SEM images of bulk (a, c) and PLD film (b, d) Ce1–xZrxO2−δ. The inset in (b) shows high-resolution SEM of CeO2−δ “island” microstructure. Images (a, c and d) are acquired in back-scattered electron contrast mode, showing a single-phase nature of the samples. The cracks observed in CeO2−δ films are expected to close at increased temperatures due to combined action of thermal and chemical expansion, inherent for ceria [29]. A thermal expansion mismatch between film and substrate Δα is estimated to be ≈ 5 ppm/K [29]. With chemical expansion coefficient of 0.1 for ceria ([38]), for the temperature increase by 600 K and non-stoichiometry of 0.01, the total expansion of an “island” is about 0.4%
In contrast to film samples, the microstructure of bulk specimens is characterized by several micrometer large particles composed of fused, about 1 µm large grains (see, e.g., Fig. 4c), and porosity,Footnote 1 especially evident in Zr-containing CZO (Fig. 4a, c).
Temperature dependence of electrical conductivity of CZO
We applied impedance spectroscopy to find out whether these microstructural features affect the electrical behavior of CZO and which phenomena are responsible for their conductivity behavior at typical operation temperatures of TWCs. Previously, the conductivity of CZO films was measured in lateral direction using the IS2 approach [29]. In this configuration, the complex impedance spectra of coarse-grained ceria typically contain well-separated frequency dispersions (semicircles) corresponding to grain interior (high-frequency domain) and grain boundary (GB; low frequency) resistances [12, 23, 24, 28, 37, 39,40,41]. As the temperature increases (or pO2 decreases), the high-frequency contributions become less distinguishable due to the exponential increase of the characteristic frequency for bulk transport with temperature [41]. Eventually, with a diminishing low-frequency part, and sometimes covered by stray capacitances, both contributions become indistinguishable essentially turning into a condensed point at the intersection with real impedance axis [28, 41]. At high temperatures, the conductivity is, thus, treated as a total conductivity [23, 28, 37, 41]. In nominally undoped ceria, it was found to be dominantly electronic [12, 24, 27, 28, 42]. In case of nanostructured materials, with decreasing grain dimensions, the GB contributions may overlap with that of the grain interiors, making it hard to distinguish them [26, 41, 43]. Furthermore, in case of films, the presence of large stray capacitances (since the volume between electrodes is small) may mask the GB-related contribution [23, 27, 28, 37]. Hence, in this work, we combined the IS4 and IS2 configurations for bulk samples to include the conditions of PLD films characterization of our previous work [29].
In Fig. 5, typical complex resistivity ρ´´(ρ´) spectra for bulk and film Ce1−xZrxO2−δ at moderate temperatures and oxidizing conditions are presented (with ρ´ and ρ´´ being the real and imaginary components of complex resistivity, respectively). For bulk CZO materials, two semicircles on the ρ´´(ρ´) plots are obtained with an IS2 approach, whereas only one semicircle is resolved with IS4 (Fig. 5a, inset of Fig. 5b). The high-frequency part (first semicircle from 0 to about 550 Ωm in Fig. 5a) of IS2 and the single semicircle from IS4 overlap, suggesting the same localization and mechanism of this charge transfer contribution in the samples. We attribute it to the grain interior conductivity (index G) σG of polycrystalline CZO with the resistance RG and a capacitance expressed by the constant phase element CPEG accounting for the non-ideal capacitive behavior of the material. The similarity of capacitances on the order of 10–12 F, as seen by the least-square fitting (LS) of the parameters of RG|CPEG (see EEC in Fig. 5a) to IS2 and IS4 spectra, supports this assumption. The RW in the depicted EEC stands for the resistance of cables and electrical connections in the equipment. Obviously, the low-frequency semicircle observed in IS2 is eliminated by the IS4 approach. Hence, the charge transfer in this frequency domain contribution can be attributed to the polarization effects at the interface between the CZO and Pt-electrodes [33, 39, 41, 44,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Since the polarization effect is related to a specific measurement approach, it will not be further evaluated and discussed. The film CZO specimens showed only one semicircle in IS2 and, apparently, no polarization-related contribution.Footnote 2 Similar results were obtained for impedance spectra of ceria-based thin films deposited with PLD onto sapphire substrates by other research groups [23, 27, 37].
Complex resistivity ρ´´(ρ´) spectra for bulk and film CeO2−δ in air (a) and for Ce1−xZrxO2−δ at different oxygen partial pressures (b). Solid lines represent the LS-fit of IS to equivalent electrical circuit (EEC) pictured in (a). The insets in (a) show the equivalent circuit without the electrode contribution that includes RW and RG|CPEG (see text, left) and the enlarged IS spectra for curves boxed in a red frame (right)
Distinguishing between the conductivity via grain interior (G) and via grain boundary (GB) is practically impossible when only one semicircle can be evaluated. Insights based on the dielectric constant of the film are not feasible, as it cannot be calculated due to the dominating stray capacitance for lateral IS measurements. Hence, in further, we analyze only the total resistance Rtot = RG + RGB of films. It is equivalent to our measured DC resistance.
The temperature dependences of σtot for films and bulk materials in air are shown in Fig. 6a. Despite the discrepancies in the absolute values of conductivities (likely, due to uncertainty of sample dimensions and porosity of bulk samples, see sec. 2) for Ce1−xZrxO2−δ with x of 0.2–0.5, the slopes in their Arrhenius representations are identical within the error margins. For Ce0.8Zr0.2O2−δ, the conductivities match. Together with linear log(σ) vs. 1/T dependences and very similar activation energies Ea for bulk and film CZO (Table 3), this observation implies that same conductance mechanism occurs in both kinds of materials between 600 and 900 °C. Moreover, the Ea values are consistent with literature data for bulk- and nano-Ce1−xZrxO2−δ [11, 12, 18, 23, 27, 48]. Based on the similarities observed for bulk and film Ce1−xZrxO2−δ (0.2 ≤ x ≤ 0.5), we conclude that the conductivity of CeO2–ZrO2 films is dominated by grain interior charge transport.
Essential observation is that for bulk Ce1−xZrxO2−δ with x ≥ 0.2, the grain conductivity component σG constitutes the total conductivity σtot of the specimens at temperatures above 500 °C (Fig. 6b), becoming a sole conductivity-limiting (i.e., dominant) factor in the concerned temperature range of 600–900 °C. In CeO2−δ, σG becomes dominant only at T > 680 °C (Fig. 6b). The observed transition is accompanied by a change in the slope of the log(σG)–1/T representation, i.e., the conductivity mechanism changes, which is indicated by a steep increase of Ea (Table 3). We have found that in Zr-containing bulk CZO the change in the slope occurred when the electrode component (second semicircle in IS2, e.g., seen only at T < 500 °C in the inset of Fig. 5b) was no longer resolved, eventually turning the spectra into a condensed point at the intersection with the ρ´-axis. For bulk CeO2−δ, these IS features occurred only at T > 700 °C or at lower pO2. According to the literature, such behavior can be ascribed to the transition from dominant ionic to dominant electronic bulk conductivity with increasing temperature [28, 41,42,43]. The evaluated high-temperature Ea values of about 2 eV for bulk CeO2−δ and 1.5–1.6 eV for Zr-containing CZO are consistent with those reported for dominant intrinsic electronic conduction of CeO2 and CZO at elevated temperatures in literature [11, 12, 24, 27, 42, 48]. Noteworthy, the conductivity activation energy includes the reduction enthalpy Hr and migration enthalpy Hm (Ea = Hr/3 + Hm), where Hm = 0.35–0.4 eV describes the small polaron hopping mechanism [11]. Thereof resulting Hr values of about 4.8 and 3.1 eV are consistent with expected reduction enthalpies for CeO2−δ and Ce1−xZrxO2−δ, respectively [11, 42]. At low temperatures (before the transition), the Ea values are much lower (Table 3). As the reduction degree at low temperatures in air is negligible [14], we suggest that in these conditions the ionic conductivity via intrinsic Frenkel defects (intrinsic oxygen vacancies formed according to \({\text{CeO}}_{2} = {\text{Ce}}_{{{\text{Ce}}}}^{ \times } + {{{\text{2V}}}}^{\bullet\bullet}_{\text{O}} \;+ \; 2\text{O}^{''}_{i}\)) and/or extrinsic oxygen vacancies introduced by acceptor (A) impurities (A2O3 = 2A′Ce + \({{\text{V}}}^{\bullet\bullet}_{\text{O}} + \; 3\text{O}^{\times}_{\text{O}}\)) dominates. In the latter case, the electroneutrality of the system requires compensation by electron holes (concentration fixed by impurity). They contribute to the electrical conductivity at temperatures below 750 °C [12]. Our Ea value of 0.78 eV for CeO2−δ is close to 0.86 eV reported in [12] for the same temperature range. As earlier reported in [29], our ceria/zirconia solid solutions were overall less pure than CeO2−δ, which may explain the lower Ea for conductivity of Ce1−xZrxO2−δ at low temperatures. In summary, we suggest that the changes in the apparent activation energies of conductivity depicted in Fig. 6b and Table 3 describe the transition from thermally activated extrinsic impurity-controlled (mixed) charge transport at low temperatures to intrinsic reduction-controlled transport at high temperatures. In the latter regime, the concentrations equation \([{\text{Ce}}_{{{\text{Ce}}}}^{\prime } \left] { = 2} \right[{{\text{V}}}^{\bullet\bullet}_{\text{O}}] = \, 2\delta\) applies, and, due to much higher mobility of electrons, the conductivity is dominantly electronic.
As stated earlier, the conductivity behavior of film and bulk Ce1−xZrxO2−δ was qualitatively similar. Meanwhile, pure ceria films show almost two orders of magnitude higher electrical conductivity and much lower Ea in contrast to bulk samples. According to the literature, this is an expected behavior explained by the impact of the higher GB density in nanostructured materials, which facilitates the charge transfer and decreases the apparent activation energy of σtot [23, 24, 26, 27, 43].
All in all, the insights from the temperature dependences of σG of CeO2 and CZO with respect to practical application point on the obvious beneficial effect of substituting Zr for Ce. That is, the addition of Zr, essentially, greatly decreases the reduction enthalpy and leads to a strongly dominating electronic conductance of Ce1−xZrxO2−δ at temperatures even about 200 K lower than for pure CeO2. Hence, the Ce1−xZrxO2−δ mixed oxides are mainly electronic conductors in a much broader temperature range than pure CeO2 and, compared to effect of Zr addition, the influence of their microstructural properties is marginal. Furthermore, since the dominant electronic conductivity in ceria/zirconia mixed oxides is related to the concentration of oxygen vacancies (oxygen non-stoichiometry δ) inside the material, these effects provide also the basis to electrically determine the actual oxygen storage level of TWC, either by direct current measurements [49] or by high-frequency methods [50], supported by the modelling conducted in [51].
Electrical conductivity of Ce1−xZrxO2−δ under reducing atmospheres
The analysis of the total electrical conductivity of Ce1−xZrxO2−δ in reducing atmospheres further supports the conclusion about the crucial effect of Zr substitution for Ce in CZO materials (Fig. 7). For both kinds of samples with 0 < x ≤ 0.67, the trends in the σG dependences on pO2 are qualitatively similar and the negative slopes obtained in the whole studied range of pO2 and at temperatures of 700–900 °C prove the dominating electronic conduction of CeO2–ZrO2. Both film and bulk CZO with x ≥ 0.2 show a classic behavior that can be derived from conventional defect chemical models of ceria and its derivatives, as described, e.g., in [11, 14, 15, 18, 52]. For discussion of our data, we introduce also small acceptor (impurity) concentrations (see sec. 2).
Electrical conductivity σtot of Ce1−xZrxO2−δ under reducing atmospheres. The correlation with non-stoichiometry data from [29] is shown for Ce0.8Zr0.2O2−δ and Ce0.5Zr0.5O2−δ. The numbers next to the curves indicate the slope, i.e., the denominator n in the σ vs (pO2)−1/n representation. The origin of hysteresis in film conductivities at moderate pO2 is described in [29]
At the highest temperature of 900 °C (Fig. 7), the variation of σG with \(\left( {p{\text{O}}_{2} } \right)^{{{-} \frac{1}{6}}}\) at moderate pO2 transits to dependences with lower exponents (hereafter, designated as \({-}\frac{1}{n}\)) at decreased oxygen potentials, which reflects the transition from intrinsic conductivity regime (dominated by small polaron hopping at \({\text{Ce}}_{{{\text{Ce}}}}^{\prime } /{\text{Ce}}_{{{\text{Ce}}}}^{ \times }\) sites [14, 15]) to a conductivity dominated by strong defect interactions, assumed to lead to the formation of either dimer \(({\text{Ce}}_{{{\text{Ce}}}}^{\prime} {{\text{V}}}^{\bullet\bullet}_{\text{O}})^{\bullet}\) [18, 52] or trimer \(({\text{Ce}}_{\text{Ce}}^{\prime}{\text{V}}_{\text{O}}^{\cdot\cdot}{\text{Ce}}_{\text{Ce}}^{\prime})^{\times}\) [16] defect associates. In the former regime, the concentration of intrinsically generated oxygen vacancies [\({{\text{V}}}^{\bullet\bullet}_{\text{O}}\)]int is much larger than that of extrinsic vacancies fixed by acceptor impurity ([\({{\text{V}}}^{\bullet\bullet}_{\text{O}}\)]ext), and electronic conductivity strongly exceeds the ionic conductivity that stem from mobile oxide ion vacancies (σGe >> σGVo) [18].
At lower pO2, the concentration of defect associates increases, leading to a σG ~ \(\left( {p{\text{O}}_{2} } \right)^{{{-} \frac{1}{n}}}\) dependence with 4 ≤ n < 6 [18, 52]. Following that, with further decreasing of oxygen partial pressure, the electrical conductivity reaches a plateau/maximum, which corresponds to the condition when concentrations of reduced and non-reduced cerium ions become equal [Ce3+] = [Ce4+] and, consequently, the small polaron hopping mechanism can no longer be supported [11]. At even further decrease in oxygen potential, [Ce3+] > [Ce4+] applies, and the conductivity decreases. This behavior is clearly evidenced at highest temperatures for Ce1−xZrxO2−δ with x = 0.5 and 0.33 (Fig. 7).
At lower temperatures (600–800 °C) and high pO2, the slope increases toward 1/4 to 1/5 with decreasing temperature. This is indicative of extrinsic \({{\text{V}}}^{\bullet\bullet}_{\text{O}}\) contribution. In this case, the conditions σGe >> σGVo and [\({{\text{V}}}^{\bullet\bullet}_{\text{O}}\)]ext >> [\({{\text{V}}}^{\bullet\bullet}_{\text{O}}\)]int apply. The conductivity is dominantly electronic, but the denominator n changes from 6 to 4 [18]. If σGe << σGVo and [\({{\text{V}}}^{\bullet\bullet}_{\text{O}}\)]int < [\({{\text{V}}}^{\bullet\bullet}_{\text{O}}\)]ext apply, the electrical conductivity is dominated by ionic transport via extrinsic \({{\text{V}}}^{\bullet\bullet}_{\text{O}}\), showing (in the extreme case of pure ionic transport) no dependence on pO2. The intermediate case, as observed here at 600–800 °C, is characterized by 4 < n < 6, where n increases with temperature [18]. In the high pO2 regime, this would correspond to an increase of [\({{\text{V}}}^{\bullet\bullet}_{\text{O}}\)]int, whereas at low pO2—to a dissociation of dimer or trimer defect complexes.
The very high values of n, observed in Ce0.33Zr0.67O2−δ at 600–800 °C for high pO2, apparently correspond to the case of σGe ≈ σGVo, where σGVo is defined by the very high and constant [\({{\text{V}}}^{\bullet\bullet}_{\text{O}}\)]ext, leading to an almost pO2-independent conductivity at 600 °C. Apparently, the same situation occurs in CeO2−δ films, with the exception that pure ceria in this study contained much less impurities than CZO [29]. While at 800–900 °C the conductivity of bulk CeO2−δ follows the classic pO2-dependence, at 600–700 °C the total conductivity σtot is less dependent on pO2 in oxidizing conditions (Fig. 8b). The σG at 600 °C is nearly independent on pO2, suggesting the conductivity via extrinsic \({{\text{V}}}^{\bullet\bullet}_{\text{O}}\). Consistent with insights from σtot(T) dependences in Fig. 6b, at 700 °C the σG of bulk CeO2−δ shows a purely electronic nature with a conventional \(\left( {p{\text{O}}_{2} } \right)^{{{-} \frac{1}{6}}}\) dependence. These two observations of σG support the earlier made assignment of dominantly electronic conductivity with the high-temperature (T > 680 °C) regime and of dominated by impurity-related extrinsic charge carriers transport with the low-temperature one (Fig. 6b) for bulk CeO2−δ. On the contrary, for CeO2−δ films, n increases with temperature at high pO2 and decreases toward 6 (slope−1/6) at the lowest oxygen activities and highest temperatures (Fig. 8a). In light of usually higher ionic contribution in σtot of nanostructured CZO [26], we assume that the observed discrepancies between bulk and PLD film ceria may be related to the smaller grain size of CeO2−δ films. The overall much higher electrical conductivity of CeO2−δ films compared to their bulk counterparts, both in σtot(T) in air and σtot(pO2) measurements, supports this assumption. Note that for another “more ionic” film material, the Ce0.33Zr0.67O2−δ, an electronic-like increase of Ea (unlike “the deceleration” of the slope for CeO2−δ) is seen in Fig. 6a, which supports the afore-mentioned assumption about the dominating influence of Zr addition over that of microstructural peculiarities in Zr-substituted ceria. The as of now available experimental results, however, do not allow us to confirm the effect of microstructure and to derive the related mechanism behind this conductivity behavior of film CeO2−δ.
Conclusion
By applying two- and four-point impedance spectroscopy, the artefactual contributions of electrode polarization effects could be successfully separated from the conductivity data for bulk and PLD film Ce1−xZrxO2−δ. It was established that in the temperature range that are typical operating conditions of automotive TWC, the CZO conductivity is dominantly electronic and is determined by the grain conductivity, whereas other possible contributions in conductivity are negligible in the temperature range of 700–900 °C. The structural peculiarities, potentially, play a significant role in the conductivity of pure nano-grained ceria films, rendering this material to behave more “ionic-like”. The substitution of Zr for Ce in CZO, apparently, does not support these contributions. The beneficial effect of Zr addition appears to dominate any possible microstructural effects on the conductivity of CZO films and drives these materials to a strongly electronic conductivity at temperatures even 200 K lower than those observed for mixed ionic/electronic to electronic conductivity transitions in bulk or film CeO2. Consequently, the film and bulk Ce1−xZrxO2−δ show qualitatively similar electrochemical behavior in reducing conditions, which could be adequately described in the framework of conventional defect model for ceria derivatives without the introduction of possible microstructural impacts.
Data availability
All relevant data presented in the article are stored according to institutional requirements and as such are not available online. However, all data used in this manuscript can be made available upon request to the authors.
Notes
The density of as-sintered bulk samples (measured by weighing method and gas pycnometer mircomeritics® AccyPyc 1330, Norcross, USA) was not lower than about 90% of theoretical density. The estimation of effective conductive volume of the samples results in conductivity correction of less than 27%. As in this study, a rather qualitative comparison of film and bulk CZO is intended, the reported conductivities are not corrected for porosity.
For bulk samples, the ratio of length to cross-sectional area is about 1 mm−1, whereas in films, it is 100–250 mm−1. Consequently, the films exhibit much higher resistances, and the interface polarization becomes negligible.
References
Trovarelli A (1996) Catalytic properties of ceria and CeO2-containing materials. Catal Rev Sci Eng 38:439–520. https://doi.org/10.1080/01614949608006464
Di Monte R, Kašpar J (2004) On the role of oxygen storage in three-way catalysis. Top Catal 28:47–57. https://doi.org/10.1023/B:TOCA.0000024333.08447.f7
Lott P, Deutschmann O (2021) Lean-burn natural gas engines: challenges and concepts for an efficient exhaust gas aftertreatment system. Emiss Control Sci Technol 7:1–6. https://doi.org/10.1007/s40825-020-00176-w
Rood S, Eslava S, Manigrasso A, Bannister C (2020) Recent advances in gasoline three-way catalyst formulation: a review. Proc Inst Mech Eng, Part D J Automobile Eng 234:936–949. https://doi.org/10.1177/0954407019859822
Sugiura M, Ozawa M, Suda A, Suzuki T, Kanazawa T (2005) Development of innovative three-way catalysts containing ceria-zirconia solid solutions with high oxygen storage/release capacity. Bull Chem Soc Jpn 78:752–767. https://doi.org/10.1246/bcsj.78.752
Moos R (2010) Catalysts as sensors—A promising novel approach in automotive exhaust gas aftertreatment. Sensors 10:6773–6787. https://doi.org/10.3390/s100706773
Kuhn M, Bishop SR, Rupp JLM, Tuller HL (2013) Structural characterization and oxygen nonstoichiometry of ceria-zirconia (Ce1-xZrxO2-δ) solid solutions. Acta Mater 61:4277–4288. https://doi.org/10.1016/j.actamat.2013.04.001
Devaiah D, Reddy LH, Park S-E, Reddy BM (2018) Ceria–zirconia mixed oxides: synthetic methods and applications. Catal Rev 60:177–277. https://doi.org/10.1080/01614940.2017.1415058
Aneggi E, Boaro M, Colussi S, De Leitenburg C, Trovarelli A (2016) Ceria-based materials in catalysis: historical perspective and future trends. In: Bünzli J-CG, Pecharsky VK (eds) Handbook on the physics and chemistry of rare earths. Elsevier, Netherlands
Andriopoulou C, Harris D, Stephenson H, Efstathiou AM, Boghosian S (2020) In situ Raman spectroscopy as a tool for discerning subtle structural differences between commercial (Ce, Zr)O2-based OSC materials of identical composition. Catalysts 10:462. https://doi.org/10.3390/catal10040462
Chen D, Cao Y, Weng D, Tuller HL (2014) Defect and transport model of ceria-zirconia solid solutions: Ce0.8Zr0.2O2−δ—An electrical conductivity study. Chem Mater 26:5143–5150. https://doi.org/10.1021/cm502565b
Chiodelli G, Flora G, Scagliotti M (1996) Electrical properties of the ZrO2–CeO2 system. Solid State Ionics 91:109–121. https://doi.org/10.1016/S0167-2738(96)00382-7
Yang Z, Woo TK, Hermansson K (2006) Effects of Zr doping on stoichiometric and reduced ceria: a first-principles study. J Chem Phys 124:224704. https://doi.org/10.1063/1.2200354
Tuller HL, Nowick AS (1979) Defect structure and electrical properties of nonstoichiometric CeO2 single crystals. J Electrochem Soc 126:209–217. https://doi.org/10.1149/1.2129007
Panhans MA, Blumenthal RN (1993) A thermodynamic and electrical conductivity study of nonstoichiometric cerium dioxide. Solid State lonics 60:279–298. https://doi.org/10.1016/0167-2738(93)90006-O
Schmitt R, Nenning A, Kraynis O, Korobko R, Frenkel AI, Lubomirsky I, Haile SM, Rupp JLM (2020) A review of defect structure and chemistry in ceria and its solid solutions. Chem Soc Rev 49:554–592. https://doi.org/10.1039/C9CS00588A
Tuller HL, Nowick AS (1977) Small polaron electron transport in reduced CeO2 single crystals. J Phys Chem Solids 38:859–867. https://doi.org/10.1016/0022-3697(77)90124-X
Izu N, Shin W, Matsubara I, Murayama N (2004) Resistive oxygen gas sensors using ceria-zirconia thick films. J Ceram Soc Jpn 112:535–539. https://doi.org/10.14852/jcersjsuppl.112.0.S535.0
Izu N, Oh-hori N, Itou M, Shin W, Matsubara I, Murayama N (2005) Resistive oxygen gas sensors based on Ce1−xZrxO2 nano powder prepared using new precipitation method. Sens Actuat B Chem 108:238–243. https://doi.org/10.1016/j.snb.2004.11.064
Izu N, Oh-hori N, Shin W, Matsubara I, Murayama N, Itou M (2008) Response of resistive oxygen sensors using Ce1-xZrxO2 (x = 0.05, 0.10) thick films in propane combustion gas. Sens Actuat B Chem 130:105–109. https://doi.org/10.1016/j.snb.2007.07.093
Melchionna M, Fornasiero P (2014) The role of ceria-based nanostructured materials in energy applications. Mater Today 17:349–357. https://doi.org/10.1016/j.mattod.2014.05.005
Hartmann P, Brezesinski T, Sann J, Lotnyk A, Eufinger J-P, Kienle L, Janek J (2013) Defect chemistry of oxide nanomaterials with high surface area: ordered mesoporous thin films of the oxygen storage catalyst CeO2–ZrO2. ACS Nano 7:2999–3013. https://doi.org/10.1021/nn400255w
Göbel MC, Gregori G, Guo X, Maier J (2010) Boundary effects on the electrical conductivity of pure and doped cerium oxide thin films. Phys Chem Chem Phys 12:14351–14361. https://doi.org/10.1039/c0cp00385a
Chiang Y-M, Lavik EB, Kosacki I, Tuller HL, Ying JY (1996) Defect and transport properties of nanocrystalline CeO2-x. Appl Phys Lett 69:185–187. https://doi.org/10.1063/1.117366
Knauth P, Engel J, Bishop SR, Tuller HL (2015) Study of compaction and sintering of nanosized oxide powders by in situ electrical measurements and dilatometry: nano CeO2-case study. J Electroceram 34:82–90. https://doi.org/10.1007/s10832-014-9946-9
Boaro M, Trovarelli A, Hwang J-H, Mason TO (2002) Electrical and oxygen storage/release properties of nanocrystalline ceria–zirconia solid solutions. Solid State Ionics 147:85–95. https://doi.org/10.1016/S0167-2738(02)00004-8
Kosacki I, Suzuki T, Petrovsky V, Anderson HU (2000) Electrical conductivity of nanocrystalline ceria and zirconia thin films. Solid State Ionics 136–137:1225–1233. https://doi.org/10.1016/S0167-2738(00)00591-9
Zhu B, Luo X, Xia C, Albinsson I, Mellander B-E (1997) Electrical properties of ZrO2-CeO2 at intermediate temperatures. Ionics 3:363–367. https://doi.org/10.1007/BF02375711
Kogut I, Wollbrink A, Steiner C, Wulfmeier H, El Azzouzi F-E, Moos R, Fritze H (2021) Linking the electrical conductivity and non-stoichiometry of thin film Ce1−xZrxO2−δ by a resonant nanobalance approach. Materials 14(4):748. https://doi.org/10.3390/ma14040748
Patterson AL (1939) The Scherrer formula for X-Ray particle size determination. Phys Rev 56:978–982. https://doi.org/10.1103/PhysRev.56.978
Pecharsky VK, Zavalij PY (2009) Fundamentals of powder diffraction and structural characterization of materials, 2nd edn. Springer, New York
McCusker LB, Von Dreele RB, Cox DE, Louër D, Scardi P (1999) Rietveld refinement guidelines. J Appl Cryst 32:36–50. https://doi.org/10.1107/S0021889898009856
Hagen G, Kita J, Izu N, Röder-Roith U, Schönauer-Kamin D, Moos R (2013) Planar platform for temperature dependent four-wire impedance spectroscopy—A novel tool for the characterization of functional materials. Sens Actuators B Chem 187:174–183. https://doi.org/10.1016/j.snb.2012.10.068
Schulz M, Fritze H, Stenzel C (2013) Measurement and control of oxygen partial pressure at elevated temperatures. Sens Actuators B Chem 187:503–508. https://doi.org/10.1016/j.snb.2013.02.115
Varez A, Garcia-Gonzalez E, Jolly J, Sanz J (2007) Structural characterization of Ce1-xZrxO2 (0 <= x <= 1) samples prepared at 1650 °C by solid state reaction. A combined TEM and XRD study. J Eur Ceram Soc 27:3677–3682. https://doi.org/10.1016/j.jeurceramsoc.2007.02.014
Meriani S, Spinolo G (1987) Powder data for metastable ZrxCe1−xO2 (x = 0.84 to 0.40) solid solutions with tetragonal symmetry. Powder Diffr 2(4):255–256. https://doi.org/10.1017/S0885715600012914
Joo JH, Choi GM (2007) Electrical conductivity of thin film ceria grown by pulsed laser deposition. J Eur Ceram Soc 27:4273–4277. https://doi.org/10.1016/j.jeurceramsoc.2007.02.135
Bishop SR, Nakamura T, Amezawa K (2014) Chemically-induced expansion of Zr0.2Ce0.8O2–δ. Solid State Ionics 261:1–4. https://doi.org/10.1016/j.ssi.2014.03.026
Koettgen J, Martin M (2020) The ionic conductivity of Sm-doped ceria. J Am Ceram Soc 103:3776–3787. https://doi.org/10.1111/jace.17066
Parras JP, Cao C, Ma Z, Mücke R, Jin L, Dunin-Borkowski R, Guillon O, De Souza RA (2019) The grain-boundary resistance of CeO2 ceramics: a combined microscopy-spectroscopy-simulation study of a dilute solution. J Am Ceram Soc 103:1755–1764. https://doi.org/10.1111/jace.16843
Souza ECC, Chueh WC, Jung W, Muccillo ENS, Haile SM (2012) Ionic and electronic conductivity of nanostructured Samaria-Doped Ceria. J Electrochem Soc 159(5):K127–K135. https://doi.org/10.1149/2.056205jes
Xiong Y-P, Kishimoto H, Yamaji K, Yoshinaga M, Horita T, Brito ME, Yokokawa H (2011) Electronic conductivity of pure ceria. Solid State Ionics 192:476–479. https://doi.org/10.1016/j.ssi.2010.07.017
Zhou X-D, Anderson HU, Huebner W (2002) Grain size and chemical composition effects on the grain boundary resistance of ceria. Mat Res Soc Symp Proc 730:44. https://doi.org/10.1557/PROC-730-V4.4
Kežionis A, Butvilas P, Šalkus T, Kazlauskas S, Petrulionis D, Žukauskas T, Kazakevičius E, Orliukas AF (2013) Four-electrode impedance spectrometer for investigation of solid ion conductors. Rev Sci Instrum 84:013902. https://doi.org/10.1063/1.4774391
Ishai PB, Talary MS, Caduff A, Levy E, Feldman Y (2013) Electrode polarization in dielectric measurements: a review. Meas Sci Technol 24:102001. https://doi.org/10.1088/0957-0233/24/10/102001
Dygas JR, Fafilek G, Breiter MW (1999) Study of grain boundary polarization by two-probe and four-probe impedance spectroscopy. Solid State Ionics 119:115–125. https://doi.org/10.1016/S0167-2738(98)00492-5
Anderson EL, Bühlmann P (2016) Electrochemical impedance spectroscopy of ion-selective membranes: artifacts in two-, three-, and four-electrode measurements. Anal Chem 88(19):9738–9745. https://doi.org/10.1021/acs.analchem.6b02641
Lee J-H, Yoon SM, Kim B-K, Lee H-W, Song HS (2002) Electrical conductivity and defect structure of CeO2-ZrO2 mixed oxide. J Mater Sci 37:1165–1171. https://doi.org/10.1023/A:1014363304942
Reiß S, Wedemann M, Moos R, Rösch M (2009) Electrical in-situ characterization of three-way catalyst coatings. Top Catal 52:1898–1902. https://doi.org/10.1007/s11244-009-9366-2
Beulertz G, Votsmeier M, Moos R (2015) Effect of propene, propane, and methane on conversion and oxidation state of three-way catalysts: a microwave cavity perturbation study. Appl Catal B-Environ 165:369–377. https://doi.org/10.1016/j.apcatb.2014.09.068
Steiner C, Walter S, Malashchuk V, Hagen G, Kogut I, Fritze H, Moos R (2020) Determination of the dielectric properties of storage materials for exhaust gas aftertreatment using the microwave cavity perturbation method. Sensors 20:6024. https://doi.org/10.3390/s20216024
Reidy RF, Simkovich G (1993) Electrical conductivity and point defect behavior in ceria-stabilized zirconia. Solid State Ionics 62:85–97. https://doi.org/10.1016/0167-2738(93)90255-2
Acknowledgements
The authors are indebted to Ms. S. Lenk for the acquisition of the SEM images and elemental analysis by EDS, to W. Dziony for high-resolution SEM characterization, and to K. Meyer for the XRD measurements (all from Clausthal University of Technology). This research was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG), Grants FR 1301/23-1 and MO 1060/29-1.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
I.K., C.S., A.W., G.H., R.M., and H.F. conceived and designed the methodology; C.S. synthesized the starting materials, fabricated the bulk CZO specimens and PLD targets, performed their XRD characterization, and measured their electrical conductivity with IS4; I.K. and A.W. prepared the film CZO specimens, performed the IS2 and DC measurements of electrical conductivity of bulk and film CZO and analyzed the data; H.W. performed the XRD characterization of film samples. All together evaluated and discussed the results and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Additional information
Handling Editor: Till Froemling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kogut, I., Steiner, C., Wulfmeier, H. et al. Comparison of the electrical conductivity of bulk and film Ce1–xZrxO2–δ in oxygen-depleted atmospheres at high temperatures. J Mater Sci 56, 17191–17204 (2021). https://doi.org/10.1007/s10853-021-06348-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06348-5