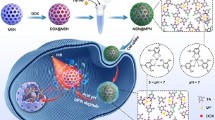
Abstract
Chemo-photothermal therapy can improve the therapeutic effects of simple chemotherapy with the help of cytotoxic heat generated by photothermal therapy. In this paper, a novel nanoplatform based on hollow mesoporous silica (HMSN) was constructed, which could achieve multi-stimuli-responsive release through chemo-photothermal interaction. Cypate as a NIR photothermal agent was conjugated to the outer surface of HMSN by disulfide bonds. Due to the insoluble feature, Cypate imparted lipophilicity to the surface of HMSN, thereby promoting the strong encapsulation of complex phospholipid layer by hydrophobic interaction. The phospholipid layer hindered the premature release of the model drug doxorubicin (DOX), prolonged the circulation time in vivo, and improved the biocompatibility and stability in physiological environment. Under glutathione, acidic conditions and NIR irradiation, the release of DOX was significantly accelerated, indicating that DOX/HMSN-CyL exhibited redox/pH/NIR multi-stimuli in response to drug release. Cytotoxicity experiments have proved that combination therapy induced the maximum levels of cell killing. Furthermore, combination index of DOX/HMSN-CyL was 0.26, demonstrating the chemo-photothermal synergetic therapy. Multicellular tumor spheroid experiments were also performed, and the same trend was showed. In summary, this novel nanoplatform has broad application prospects as a drug delivery system for combination therapy.







Similar content being viewed by others
References
Chen W, Zhong P, Meng F, Cheng R, Deng C, Feijen J et al (2013) Redox and pH-responsive degradable micelles for dually activated intracellular anticancer drug release. J Control Release 169:171–179
Kim T-W, Slowing II, Chung P-W, Lin VS-Y (2010) Ordered Mesoporous polymer−silica hybrid nanoparticles as vehicles for the intracellular controlled release of macromolecules. ACS Nano 5:360–366
Lee CH, Cheng SH, Huang IP, Souris JS, Yang CS, Mou CY et al (2010) Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew Chem Int Ed 49:8214–8219
Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z (2011) Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release 152:2–12
Cui Y, Dong H, Cai X, Wang D, Li Y (2012) Mesoporous silica nanoparticles capped with disulfide-linked PEG gatekeepers for glutathione-mediated controlled release. ACS Appl Mater Interfaces 4:3177–3183
Wang J, Sun X, Mao W, Sun W, Tang J, Sui M et al (2013) Tumor redox heterogeneity-responsive prodrug nanocapsules for cancer chemotherapy. Adv Mater 25:3670–3676
Luo C, Sun J, Liu D, Sun B, Miao L, Musetti S et al (2016) Self-assembled redox dual-responsive prodrug-nanosystem formed by single thioether-bridged paclitaxel-fatty acid conjugate for cancer chemotherapy. Nano Lett 16:5401–5408
Zou Z, He X, He D, Wang K, Qing Z, Yang X et al (2015) Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials 58:35–45
Liang L, Lin S-W, Dai W, Lu J-K, Yang T-Y, Xiang Y et al (2012) Novel cathepsin B-sensitive paclitaxel conjugate: Higher water solubility, better efficacy and lower toxicity. J Control Release 160:618–629
Lu J, Choi E, Tamanoi F, Zink JI (2008) Light-activated nanoimpeller-controlled drug release in cancer cells. Small 4:421–426
Yang G, Sun X, Liu J, Feng L, Liu Z (2016) Light-responsive, singlet-oxygen-triggered on-demand drug release from photosensitizer-doped mesoporous silica nanorods for cancer combination therapy. Adv Funct Mater 26:4722–4732
Xiao W, Chen WH, Xu XD, Li C, Zhang J, Zhuo RX et al (2011) Design of a cellular-uptake-shielding “plug and play” template for photo controllable drug release. Adv Mater 23:3526–3530
Fang W, Tang S, Liu P, Fang X, Gong J, Zheng N (2012) Pd nanosheet-covered hollow mesoporous silica nanoparticles as a platform for the chemo-photothermal treatment of cancer cells. Small 8:3816–3822
Liu Y, Ai K, Liu J, Deng M, He Y, Lu L (2013) Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater 25:1353–1359
Lee S-M, Kim HJ, Kim SY, Kwon M-K, Kim S, Cho A et al (2014) Drug-loaded gold plasmonic nanoparticles for treatment of multidrug resistance in cancer. Biomaterials 35:2272–2282
Pan Y, Le Z, Zeng L, Ren W, Xiao X, Zhang J et al (2016) Gd-based upconversion nanocarriers with yolk–shell structure for dual-modal imaging and enhanced chemotherapy to overcome multidrug resistance in breast cancer. Nanoscale 8:878–888
Xing Y, Zhang J, Chen F, Liu J, Cai K (2017) Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale 9:8781–8790
Li Y, Xu X, Zhang X, Li Y, Zhang Z, Gu Z (2016) Tumor-specific multiple stimuli-activated dendrimeric nanoassemblies with metabolic blockade surmount chemotherapy resistance. ACS Nano 11:416–429
Zhang Z, Wang J, Chen C (2013) Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv Mater 25:3869–3880
Deng Y, Käfer F, Chen T, Jin Q, Ji J, Agarwal S (2018) Let there be light: polymeric micelles with upper critical solution temperature as light-triggered heat nanogenerators for combating drug-resistant cancer. Small 14:1802420
Yu H, Cui Z, Yu P, Guo C, Feng B, Jiang T et al (2015) pH- and NIR light-responsive micelles with hyperthermia-triggered tumor penetration and cytoplasm drug release to reverse doxorubicin resistance in breast cancer. Adv Funct Mater 25:2489–2500
Liu Q, Guo B, Rao Z, Zhang B, Gong JR (2013) Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett 13:2436–2441
Wu X, Ming T, Wang X, Wang P, Wang J, Chen J (2009) High-photoluminescence-yield gold nanocubes: for cell imaging and photothermal therapy. ACS Nano 4:113–120
Yang K, Zhang S, Zhang G, Sun X, Lee S-T, Liu Z (2010) Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett 10:3318–3323
Su S, Ding Y, Li Y, Wu Y, Nie G (2016) Integration of photothermal therapy and synergistic chemotherapy by a porphyrin self-assembled micelle confers chemosensitivity in triple-negative breast cancer. Biomaterials 80:169–178
Deng Y, Huang L, Yang H, Ke H, He H, Guo Z et al (2017) Cyanine-Anchored Silica Nanochannels for Light-Driven Synergistic Thermo-Chemotherapy. Small 13:1602747
Wang L, Kim M, Fang Q, Min J, Jeon WI, Lee SY et al (2013) Hydrophobic end-gated silica nanotubes for intracellular glutathione-stimulated drug delivery in drug-resistant cancer cells. Chem Commun 49:3194–3196
Treger JS, Priest MF, Iezzi R, Bezanilla F (2014) Real-time imaging of electrical signals with an infrared FDA-approved dye. Biophys J 107:L09–L12
Fu C, Liu T, Li L, Liu H, Chen D, Tang F (2013) The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 34:2565–2575
Liu T, Li L, Teng X, Huang X, Liu H, Chen D et al (2011) Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials 32:1657–1668
Ye Y, Li WP, Anderson CJ, Kao J, Nikiforovich GV, Achilefu S (2003) Synthesis and characterization of a macrocyclic near-infrared optical scaffold. J Am Chem Soc 125:7766–7767
Fang X, Chen C, Liu Z, Liu P, Zheng N (2011) A cationic surfactant assisted selective etching strategy to hollow mesoporous silica spheres. Nanoscale 3:1632–1639
Zhao Q, Liu J, Zhu W, Sun C, Di D, Zhang Y et al (2015) Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater 23:147–156
Ebright YW, Chen Y, Kim Y, Ebright RH (1996) S-[2-(4-A zidosalicylamido) ethylthio]-2-thiopyridine: radioiodinatable, cleavable, photoactivatible cross-linking agent. Bioconjugate Chem 7:380–384
Jiao J, Li X, Zhang S, Liu J, Di D, Zhang Y et al (2016) Redox and pH dual-responsive PEG and chitosan-conjugated hollow mesoporous silica for controlled drug release. Mater Sci Eng C 67:26–33
Tina U, Aljaz G, Odon PE, Venčeslav KI, Gregor M, Miran GE (2011) The phase (trans)formation and physical state of a model drug in mesoscopic confinement. Phys Chem Chem Phys 13:16046–16054
Tang H, Guo J, Sun Y, Chang B, Ren Q, Yang W (2011) Facile synthesis of pH sensitive polymer-coated mesoporous silica nanoparticles and their application in drug delivery. Int J Pharm 421:388–396
Yufang Z, Toshiyuki I, Nobutaka H, Stefan K (2010) Rattle-type Fe3O4@SiO2 hollow mesoporous spheres as carriers for drug delivery. Small 6:471–478
Shen S, Tang H, Zhang X, Ren J, Pang Z, Wang D et al (2013) Targeting mesoporous silica-encapsulated gold nanorods for chemo-photothermal therapy with near-infrared radiation. Biomaterials 34(12):3150–3158
Zhou L, Jing Y, Liu Y, Liu Z, Gao D, Chen H et al (2018) Mesoporous carbon nanospheres as a multifunctional carrier for cancer theranostics. Theranostics 8(3):663
Wang LS, Wu LC, Lu SY, Chang LL, Teng IT, Yang CM et al (2010) Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: improved water suspensibility and decreased nonspecific protein binding. ACS Nano 4:4371–4379
Moghimi SM, Hunter AC, Murray JC (2005) Nanomedicine: current status and future prospects. Faseb J Off Publ Feder Am Soc Exp Biol 19:311–330
Slowing II, Chia-Wen W, Vivero-Escoto JL, Victor S-YL (2010) Mesoporous silica nanoparticles for reducing hemolytic activity towards mammalian red blood cells. Small 5:57–62
Tian Y, Alexander M, Hamidreza G (2011) Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano 5:5717–5728
Zhao Q, Yang Y, Wang H, Lei W, Liu Y, Wang S (2019) Gold nanoparticles modified hollow carbon system for dual-responsive release and chemo-photothermal synergistic therapy of tumor. J Colloid Interface Sci 554:239–249
Feng S, Mao Y, Wang X, Zhou M, Lu H, Zhao Q et al (2020) Triple stimuli-responsive ZnO quantum dots-conjugated hollow mesoporous carbon nanoplatform for NIR-induced dual model antitumor therapy. J Colloid Interface Sci 559:51–64
Owens EA, Hyun H, Tawney JG, Choi HS, Henary M (2015) Correlating molecular character of NIR imaging agents with tissue-specific uptake. J Med Chem 58(10):4348–4356
Cheng L, Wang C, Feng L, Yang K, Liu Z (2014) Functional nanomaterials for phototherapies of cancer. Chin J Clin Oncol 114(21):10869–10939
Yang J, Su H, Sun W, Cai J, Liu S, Chai Y et al (2018) Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics 8:1966–1984
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 81603058 and 81473165) and Career Development Program for Young Teachers in Shenyang Pharmaceutical University (No. ZQN2016025).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Y., Lu, J., Mao, Y. et al. Composite phospholipid-coated hollow mesoporous silica nanoplatform with multi-stimuli responsiveness for combined chemo-photothermal therapy. J Mater Sci 55, 5230–5246 (2020). https://doi.org/10.1007/s10853-019-04314-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04314-w