Abstract
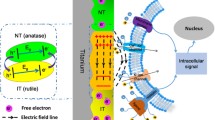
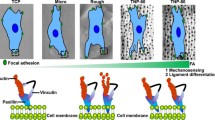
The current trends in bone tissue engineering aim to fasten the cells osteogenic differentiation by mechanical stimulation. To date, several approaches have proved efficient for this purpose. One is related to changing the shape of the cells nuclei using topological surfaces with appropriate dimensions and stiffness. Another successful method is by low-intensity pulsed ultrasound stimulation (LIPUS) of the cells. The goal of this proof-of-concept study is to introduce and validate, for the first time, the synergistic effect of topological surfaces and LIPUS for improving the osteogenic differentiation of osteoblast-like cells. Cells were grown on topological surfaces consisting of vertical microtubes fabricated by laser direct writing. The flexibility of the topological surfaces was tuned by varying the microtubes’ height. The spatial arrangement and dimensions of the microtubes limited the cell–cell interactions and allowed us to observe individual cells. A finite element model simulation was proposed for explaining the cell–surface interaction details. We monitored the cells nuclei deformations in response to the topological surfaces in conjunction with LIPUS. The topological surfaces alone induced dramatic changes of the shape of the cells nuclei that wrapped around the microtubes. The nuclei deformation was further increased by LIPUS. This synergy between the topological surfaces and LIPUS allowed us to obtain an increase of up to 200% in the cells osteogenic differentiation, as determined by ALP activity and osteocalcin secretion measurements, in comparison with flat surfaces in static regime. A causal relationship between the nuclei deformation and the cells osteogenic differentiation was established.









Similar content being viewed by others
References
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40:363–408. https://doi.org/10.1615/CritRevBiomedEng.v40.i5.10
Liu X, Liu R, Gu Y, Ding J (2017) Non-monotonic self-deformation of cell nuclei on topological surfaces with micropillar array interfaces. ACS Appl Mater Interfaces 9:18521–18530. https://doi.org/10.1021/acsami.7b04027
Yim EK, Pang SW, Leong KW (2007) Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res 313:1820–1829. https://doi.org/10.1016/j.yexcr.2007.02.031
Qu ZH, Ding JD (2012) Sugar-fiber imprinting to generate microgrooves on polymeric film surfaces for contact guidance of cells. Chin J Chem 30:2292–2296. https://doi.org/10.1002/cjoc.201200841
Fu X, Xu M, Liu J, Qi Y, Li S, Wang H (2014) Regulation of migratory activity of human keratinocytes by topography of multiscale collagen-containing nanofibrous matrices. Biomaterials 35:1496–1506. https://doi.org/10.1016/j.biomaterials.2013.11.013
Pan Z, Duan PG, Liu XN, Wang HR, Cao L, He Y, Dong J, Ding JD (2015) Effect of porosities of bilayered porous scaffolds on spontaneous osteochondral repair in cartilage tissue engineering. Regener Biomater 2:9–19. https://doi.org/10.1093/rb/rbv001
Jahed Z, Zareian R, Chau YY, Seo BB, West M, Tsui TY, Wen W, Mofrad MR (2016) Differential collective- and single-cell behaviors on silicon micropillar arrays. ACS Appl Mater Interfaces 8:23604–23613. https://doi.org/10.1021/acsami.6b08668
Wang S, Wan Y, Liu Y (2014) Effects of nanopillar array diameter and spacing on cancer cell capture and cell behaviors. Nanoscale 6:12482–12489. https://doi.org/10.1039/c4nr02854f
Xie C, Hanson L, Xie W, Lin Z, Cui B, Cui Y (2010) Noninvasive neuron pinning with nanopillar arrays. Nano Lett 10:4020–4024. https://doi.org/10.1021/nl101950x
Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS (2010) Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods 7:733–736. https://doi.org/10.1038/nmeth.1487
Koch B, Sanchez S, Schmidt CK, Swiersy A, Jackson SP, Schmidt OG (2014) Confinement and deformation of single cells and their nuclei inside size-adapted microtubes. Adv Healthc Mater 3:1753–1758. https://doi.org/10.1002/adhm.201300678
Dimitriou R, Babis GC (2007) Biomaterial osseointegration enhancement with biophysical stimulation. J Musculoskelet Neuronal Interact 7:253–265
Liu Q, Liu X, Liu B, Hu K, Zhou X, Ding Y (2012) The effect of low-intensity pulsed ultrasound on the osseointegration of titanium dental implants. Br J Oral Maxillofac Surg 50:244–250. https://doi.org/10.1016/j.bjoms.2011.03.001
Paun IA, Zamfirescu M, Luculescu CR, Acasandrei AM, Mustaciosu CC, Mihailescu M, Dinescu M (2017) Electrically responsive microreservoires for controllable delivery of dexamethasone in bone tissue engineering. Appl Surf Sci 392:321–331. https://doi.org/10.1016/j.apsusc.2016.09.027
Paun IA, Popescu RC, Mustaciosu CC, Zamfirescu M, Calin BS, Mihailescu M, Dinescu M, Popescu A, Chioibasu D, Soproniy M, Luculescu CR (2018) Laser-direct writing by two-photon polymerization of 3D honeycomb-like structures for bone regeneration. Biofabrication 10:025009. https://doi.org/10.1088/1758-5090/aaa718
Paun IA, Popescu RC, Calin BS, Mustaciosu CC, Dinescu M, Luculescu CR (2018) 3D biomimetic magnetic structures for static magnetic field stimulation of osteogenesis. Int J Mol Sci 19:495–513. https://doi.org/10.3390/ijms19020495
Romano CL, Romano D, Logoluso N (2009) Low-intensity pulsed ultrasound for the treatment of bone delayed union or nonunion: a review. Ultrasound Med Biol 35:529–536. https://doi.org/10.1016/j.ultrasmedbio.2008.09.029
Escoffre JM, Bouakaz A (eds) (2016) Therapeutic ultrasound. Springer, Belrin. https://doi.org/10.1007/978-3-319-22536-4_1
Rosenblum J, Rosenblum S, Karpf A (2017) Low intensity low frequency ultrasound surface acoustic wave treatment for metatarsal fractures. J Sports Med Doping Stud. https://doi.org/10.4172/2161-0673.1000193
Harrison A, Lin S, Pounder N, Mikuni-Takagaki Y (2016) Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics 70:45–52. https://doi.org/10.1016/j.ultras.2016.03.016
FA Duck (1990) Physical properties of tissue: a comprehensive reference book. Academic Press, London. https://doi.org/10.1016/B978-0-12-222800-1.50009-7
Padilla F, Puts R, Vico L, Raum K (2014) Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics 54:1125–1145. https://doi.org/10.1016/j.ultras.2014.01.004
Jin Y, Zhang Y, Ouyang H, Peng M, Zhai J, Li Z (2015) Quantification of cell traction force of osteoblast cells using Si nanopillar-based mechanical sensor. Sens Mater 27:1071–1077. https://doi.org/10.18494/sam.2015.1144
Schroer A, Bauer J, Schwaiger R, Kraft O (2016) Optimizing the mechanical properties of polymer resists for strong and light-weight micro-truss structures. Extreme Mech Lett 8:283–291. https://doi.org/10.1016/j.eml.2016.04.014
Bandow K, Nishikawa Y, Ohnishi T, Kakimoto K, Soejima K, Iwabuchi S, Kuroe K, Matsuguchi T (2007) Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and MIP-1beta expression in osteoblasts through the angiotensin II type 1 receptor. J Cell Physiol 211:392–398. https://doi.org/10.1002/jcp.20944
Suzuki A, Takayama T, Suzuki N, Sato M, Fukuda T, Ito K (2009) Daily low-intensity pulsed ultrasound-mediated osteogenic differentiation in rat osteoblasts. Acta Biochim Biophys Sin 41:108–115
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469. https://doi.org/10.1016/j.cell.2007.05.047
Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A (2012) Engineering microscale topographies to control the cell–substrate interface. Biomaterials 33:5230–5246. https://doi.org/10.1016/j.biomaterials.2012.03.079
Jiang X, Savchenko O, Li Y, Qi S, Yang T, Zhang W, Chen J (2018) A review of low-intensity pulsed ultrasound for therapeutic applications. IEEE Trans Biomed Eng. https://doi.org/10.1109/TBME.2018.2889669
Man J, Shelton RM, Cooper PR, Landini G, Scheven BA (2012) Low intensity ultrasound stimulates osteoblast migration at different frequencies. J Bone Miner Metab 30:602–607. https://doi.org/10.1007/s00774-012-0368-y
Reher P, Doan N, Bradnock B, Meghji S, Harris M (1999) Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine 11:416–423. https://doi.org/10.1006/cyto.1998.0444
Reher P, Doan N, Bradnock B, Meghji S, Harris M (1998) Therapeutic ultrasound for osteoradionecrosis: an in vitro comparison between 1 MHz and 45 kHz machines. Eur J Cancer 34:1962–1968. https://doi.org/10.1016/S0959-8049(98)00238-X
Purtov J, Verch A, Rogin P, Hensel R (2018) Improved development procedure to enhance the stability of microstructures created by two-photon polymerization. Microelectron Eng 194:45–50. https://doi.org/10.1016/j.mee.2018.03.009
Haase K, Macadangdang JKL, Edrington CH, Cuerrier CM, Hadjiantoniou S, Harden JL, Skerjanc IS, Pelling AE (2016) Extracellular forces cause the nucleus to deform in a highly controlled anisotropic manner. Sci Rep 6:21300. https://doi.org/10.1038/srep21300
Versaevel M, Grevesse T, Gabriele S (2012) Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat Commun 3:671. https://doi.org/10.1038/ncomms1668
Marino A, Filippeschi C, Genchi GC, Mattoli V, Mazzolai B, Ciofani G (2014) The osteoprint: a bioinspired two-photon polymerized 3-D structure for the enhancement of bone-like cell differentiation. Acta Biomater 10:4304–4313. https://doi.org/10.1016/j.actbio.2014.05.032
Rego EB, Takata T, Tanne K, Tanaka E (2012) Current status of low intensity pulsed ultrasound for dental purposes. Open Dent J 6:220–225. https://doi.org/10.2174/1874210601206010220
Rutten S, Nolte PA, Guit GL, Bouman DE, Albers GH (2007) Use of low-intensity pulsed ultrasound for posttraumatic nonunions of the Tibia: a review of patients treated in the Netherlands. J Trauma 62:902–908. https://doi.org/10.1097/01.ta.0000238663.33796.fb
Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM, Greenleaf JF (1994) Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthop Res 12:40–47. https://doi.org/10.1002/jor.1100120106
Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF (1994) Acceleration of tibial fracture-healing by noninvasive, low-intensity pulsed ultrasound. J Bone Jt Surg 76:26–34. https://doi.org/10.2106/00004623-199401000-00004
Zheng H, Lu L, Song JL, Gao X, Deng F, Wang ZB (2011) Low intensity pulsed ultrasound combined with guided tissue regeneration for promoting the repair of defect at canines periodontal fenestration in beagle dogs. Zhonghua Kouqiang Yixue Zazhi 46:431–436
Lim K, Kim J, Seonwoo H, Park SH, Choung PH, Chung JH (2013) In vitro effects of low-intensity pulsed ultrasound stimulation on the osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells for tooth tissue engineering. Biomed Res Int 2013:269724. https://doi.org/10.1155/2013/269724
Acknowledgements
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS/CCCDI—UEFISCDI, project number PN-III-P2-2.1-PED-2016-1787. A part of this work was supported by the National Program LAPLAS VI (16N/08.02.2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2019_3680_MOESM1_ESM.docx
We provide supplementary information concerning: 1) analysis of the fluorescence images of the cells nuclei using a code built in MATLAB; 2) Finite Element Model simulation performed in SolidWorks® that evaluates the microtubes’ deformation at the contact with the cells; 3) normal vibration modes of the microtubes and of the cells on microtubes; 4) scanning electron micrographs of topological surfaces fabricated by laser direct writing via two photon-polymerization of IP-L780 photopolymer, with microtubes heights above 20 μm; 5) wide area scanning electron micrograph of topological surface fabricated by laser direct writing via two-photon polymerization of IP-L780 photopolymer, for microtubes height of 20 μm (DOCX 6887 kb)
Rights and permissions
About this article
Cite this article
Paun, I.A., Calin, B.S., Mustaciosu, C.C. et al. Osteogenic cells differentiation on topological surfaces under ultrasound stimulation. J Mater Sci 54, 11213–11230 (2019). https://doi.org/10.1007/s10853-019-03680-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03680-9