Abstract
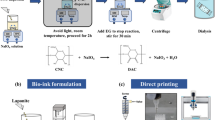
Three-dimensional (3D) printing, used to fabricate modular and patient-specific scaffolds with high structural complexity and design flexibility, has drawn wide attentions in the tissue engineering area. However, one of the key problems hindering the application and development of 3D printing in TE area is the poor mechanical property of bio-inks. In this work, we aimed to design a high-strength hydrogel system based on dialdehyde cellulose nanocrystals (DAC) and gelatin (GEL) as a new bio-ink for 3D printing of scaffolds. The DAC were prepared and used as a natural crosslinker to interact with the GEL through a Schiff base reaction. The mechanical test results indicated that the breaking strength of the optimal 4:8-DAC/GEL sample was almost 41.3-fold greater than that of the GEL hydrogel. According to the rheological test results, the 4:8-DAC/GEL sample incubated for 3 h was proposed as a bio-ink for 3D printing. Then, the printing conditions, including the printing pressure and nozzle speed, as well as additional crosslinking conditions for a freshly printed scaffold, i.e., the crosslinking time and temperature, were optimized. Crosslinked scaffolds with adjustable porosity and good fidelity were successfully obtained. The biocompatibility of 4:8-DAC/GEL was also investigated by CCK-8 and Hoechst 33342/PI double-staining assays. Collectively, these results confirm the good potential of the 4:8-DAC/GEL hydrogel as a 3D bio-ink for application in tissue repair.







Similar content being viewed by others
References
Zhang YS, Yue K, Aleman J et al (2017) 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng 45(1):148–163
Zhang X, Yang Y, Yao J, Shao Z, Chen X (2014) Strong collagen hydrogels by oxidized dextran modification. ACS SustaiChem Eng 2(5):1318–1324
Li H, Liu S, Lin L (2016) Rheological study on 3D printability of alginate hydrogel and effect of graphene oxide. Int J Bioprinting 2(2):54–66
Abbadessa A, Blokzijl MM, Mouser VH et al (2016) A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3d printing applications. Carbohydr Polym 149:163–174
Christensen K, Xu C, Chai W, Zhang Z, Fu J, Huang Y (2015) Freeform inkjet printing of cellular structures with bifurcations. Biotechnol Bioeng 112(5):1047–1055
Duan B, Hockaday LA, Kang KH et al (2013) 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res Part A 101A(5):1255–1264
Colosi C, Shin SR, Manoharan V et al (2016) Microfluidic bioprinting of heterogeneous 3d tissue constructs using low viscosity bioink. Adv Mater 28(4):677–681
Lee VK, Kim DY, Ngo H et al (2014) Creating perfused functional vascular channels using 3d bio-printing technology. Biomaterials 35(28):8092–8102
Kim Y, Lee H, Kim G (2016) Strategy to achieve highly porous/biocompatible macroscale cell blocks, using a collagen/genipin-bioink and an optimal 3D printing process. ACS Appl Mater Interfaces 8(47):32230–32240
Aleksander S, David M, Edi K et al (2012) Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 1(11):792–802
Highley CB, Rodell CB, Burdick JA (2015) Direct 3d printing of shear-thinning hydrogels into self-healing hydrogels. Adv Mater 27(34):5075–5079
Muller WEG, Neufurth M, Tolba E et al (2015) A new printable and durable n, o-carboxymethyl chitosan-ca2 + -polyphosphate complex with morphogenetic activity. J Mater Chem B 3(8):1722–1730
Zhang X, Yang Y, Yao J et al (2014) Strong collagen hydrogels by oxidized dextran modification. Acs Sustain Chem Eng 2(5):1318–1324
Tan YJ, Tan X, Yeong WY et al (2016) Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: a new biofabrication strategy. Sci Rep 6:39140
Kim YB, Lee H, Kim GH (2016) Strategy to achieve highly porous/biocompatible macroscale cell blocks, using a collagen/genipin-bioink and an optimal 3D printing process. Acs Appl Mater Interfaces 8(47):32230–32240
Kim T, Sridharan I, Zhu B, Effect of CNT on collagen fiber structure et al (2015) Stiffness assembly kinetics and stem cell differentiation. Mater Sci Eng C Mater Biol Appl 49:281–289
Peppas N, Hilt J, Khademhosseini A et al (2006) Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater 18(11):1345–1360
Henriksson I, Gatenholm P, Hägg DA (2017) Increased lipid accumulation and adipogenic gene expression of adipocytes in 3D bioprinted nanocellulose scaffolds. Biofabrication 9(1):015022
Piras CC, Fernándezprieto S, De Borggraeve WM (2017) Nanocellulosic materials as bioinks for 3D bioprinting. Biomater Sci 5:1988–1992
Murphy CA, Collins MN (2016) Microcrystalline cellulose reinforced polylactic acid biocomposite filaments for 3D printing. Polym Compos 15:236–244
Nguyen D, Hägg DA, Forsman A et al (2017) Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Reports 7(1):658
Markstedt K, Mantas A, Tournier I et al (2015) 3D bioprinting human chondrocytes with nanocellulose alginate bioink for cartilage tissue engineering applications. Biomacromol 16(5):1489–1496
Ávila HM, Schwarz S, Rotter N et al (2016) 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 1(2):22–35
Müller M, Öztürk E, Arlov O et al (2016) Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications. Ann Biomed Eng 45(1):210–223
Sawkins MJ, Mistry P, Brown BN et al (2015) Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair. Biofabrication 7(3):035004
Torres-Rendon JG, Koepf M, Gehlen D et al (2016) Cellulose nanofibril hydrogel tubes as sacrificial templates for freestanding tubular cell constructs. Biomacromol 17:905–913
Siqueira G, Kokkinis D, Libanori R et al (2017) Cellulose nanocrystal inks for 3D printing of textured cellular architectures. Adv Funct Mater 27(12):1604619
Lu T, Li Q, Chen W, Yu H (2014) Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos Sci Technol 94(4):132–138
Jiang Y, Zhou J, Zhang Q et al (2017) Preparation of cellulose nanocrystals from humulus japonicus stem and the influence of high temperature pretreatment. Carbohydr Polym 64:284–293
Iii CMO, Bubnis WA (1996) Chemical and swelling evaluations of amino group crosslinking in gelatin and modified gelatin matrices. Pharm Res 13(12):1821–1827
Zhang R, Ma PX (1999) Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering I preparation and morphology. J Biomed Mater Res 44(4):446–455
Chen W, Yu H, Li Q, Liu Y, Li J (2011) Ultralight and highly flexible aerogels with long cellulose nanofibers. Soft Matter 7(21):10360–10368
Sain M, Panthapulakkal S (2006) Bioprocess preparation of wheat straw fibers and their characterization. Ind Crops Prod 23(1):1–8
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues-wheat straw and soy hulls. Biores Technol 99(6):1664–1671
Münster L, Vícha J, Klofáč J, Masař M, Kucharczyk P, Kuřitka I (2017) Stability and aging of solubilized dialdehyde cellulose. Cellulose 24(7):1–14
Pietrucha K, Safandowska M (2015) Dialdehyde cellulose-crosslinked collagen and its physicochemical properties. Process Biochem 50(12):2105–2111
Dorris GM, Gray DG (1978) Surface analysis of paper and wood fibres by ESCA II surface composition of mechanical pulps. Cellul Chem Technol 12:721–734
Gao C, Yan T, Dai K, Wan Y (2012) Immobilization of gelatin onto natural nanofibers for tissue engineering scaffold applications without utilization of any crosslinking agent. Cellulose 19(3):761–768
Hayami JWS, Waldman SD, Amsden BG (2015) Photo-cross-linked methacrylated polysaccharide solution blends with high chondrocyte viability, minimal swelling, and moduli similar to load bearing soft tissues. Eur Polym J 72:687–697
Das S, Pati F, Choi YJ et al (2015) Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage dif` ferentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater 11(1):233–246
Wei LN, Yeong WY, Naing MW (2016) Polyelectrolyte gelatin-chitosan hydrogel optimized for 3d bioprinting in skin tissue engineering. Int J Bioprinting 2(1):53–62
Yashima S, Takase N, Kurokawa T, Gong JP (2014) Friction of hydrogels with controlled surface roughness on solid flat substrates. Soft Matter 10(18):3192–3199
Kanth SV, Ramaraj A, Rao JR, Nair BU (2015) Stabilization of type I collagen using dialdehyde cellulose. Process Biochem 4:869–874
Huang S, Yao B, Xie J, Fu X (2016) 3d bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater 32:170–177
Zhang S, Huang Y, Yang X et al (2009) Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J Biomed Mater Res Part A 90(3):671–679
Acknowledgements
This research was supported by the Excellent Doctoral Thesis Support Program of Yangzhou University, the City and School Cooperation Project, the Top Talents Support Program of Yangzhou University, the Natural Science Foundation of China (81770018).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Y., Zhou, J., Yang, Z. et al. Dialdehyde cellulose nanocrystal/gelatin hydrogel optimized for 3D printing applications. J Mater Sci 53, 11883–11900 (2018). https://doi.org/10.1007/s10853-018-2407-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2407-0