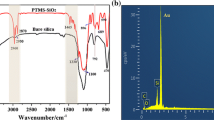
Abstract
To fabricate a superhydrophobic and durable coating, non-fluorine siloxane solution for the coating was successfully synthesized via a facile one-pot method. The wetting properties and morphology of coatings with different concentration and adding time of additives were studied. Under condition of low water content, the coatings’ superhydrophobicity was improved with the increasing amount of ammonia content, water content, and low-surface-energy material (HTMS) content. However, too more ammonia or water or HTMS would sacrifice the coating’s integrality and lead to the cracking of it. The obtained coatings showed good superhydrophobicity and anti-UV property on plain and even curved substrates, including glass, metal, and polymer. Contact angle and sliding angle of the coating could reach 158° and 1.8°, respectively. What is more, coating on PET filter could be used for oil/water separation with an efficiency of 80%.










Similar content being viewed by others
References
Wang S, Jiang L (2007) Definition of superhydrophobic states. Adv Mater 19(21):3423–3424
Marmur A (2004) The lotus effect: superhydrophobicity and metastability. Langmuir 20(9):3517–3519
Wang Y, Xue J, Wang Q, Chen Q, Ding J (2013) Verification of icephobic/anti-icing properties of a superhydrophobic surface. ACS Appl Mater Interfaces 5(8):3370–3381
Wang Z, Li Q, She Z, Chen F, Li L (2012) Low-cost and large-scale fabrication method for an environmentally-friendly superhydrophobic coating on magnesium alloy. J Mater Chem 22(9):4097–4105
Xiu Y, Hess DW, Wong CP (2008) UV and thermally stable superhydrophobic coatings from sol–gel processing. J Colloid Interface Sci 326(2):465–470
Tian Y, Guo K, Bian X, Sun J (2017) Durable and room-temperature curable superhydrophobic composite coating on nitrocellulose lacquer. Surf Coat Technol 325:444–450
Ju J, Xiao K, Yao X, Bai H, Jiang L (2013) Bioinspired conical copper wire with gradient wettability for continuous and efficient fog collection. Adv Mater 25(41):5937–5942
Zimmermann J, Reifler FA, Schrade U, Artus GRJ, Seeger S (2007) Long term environmental durability of a superhydrophobic silicone nanofilament coating. Colloids Surf A Physicochem Eng Asp 302(1–3):234–240
Chen K, Zhou S, Yang S, Wu L (2015) Fabrication of all-water-based self-repairing superhydrophobic coatings based on UV-responsive microcapsules. Adv Funct Mater 25(7):1035–1041
Zheng S, Li C, Fu Q et al (2016) Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater Des 93:261–270
Su B, Tian Y, Jiang L (2016) Bioinspired interfaces with superwettability: from materials to chemistry. J Am Chem Soc 138(6):1727–1748
Blossey R (2003) Self-cleaning surfaces—virtual realities. Nat Mater 2(5):301–306
Feng X, Jiang L (2006) Design and creation of superwetting/antiwetting surfaces. Adv Mater 18(23):3063–3078
Gao L, McCarthy TJ (2006) The “lotus effect” explained: two reasons why two length scales of topography are important. Langmuir 22(7):2966–2967
Yan YY, Gao N, Barthlott W (2011) Mimicking natural superhydrophobic surfaces and grasping the wetting process: a review on recent progress in preparing superhydrophobic surfaces. Adv Colloid Interface Sci 169(2):80–105
Deng X, Mammen L, Butt H-J, Vollmer D (2012) Candle soot as a template for a transparent robust superamphiphobic coating. Science 335(6064):67–70
Zhang L, Wu J, Wang Y, Long Y, Zhao N, Xu J (2012) Combination of bioinspiration: a general route to superhydrophobic particles. J Am Chem Soc 134(24):9879–9881
Du X, Liu X, Chen H, He J (2009) Facile fabrication of raspberry-like composite nanoparticles and their application as building blocks for constructing superhydrophilic coatings. J Phys Chem C 113(21):9063–9070
Cortese B, D’Amone S, Manca M, Viola I, Cingolani R, Gigli G (2008) Superhydrophobicity due to the hierarchical scale roughness of PDMS surfaces. Langmuir 24(6):2712–2718
Lv T, Cheng Z, Zhang E, Kang H, Liu Y, Jiang L (2016) Self-restoration of superhydrophobicity on shape memory polymer arrays with both crushed microstructure and damaged surface chemistry. Small 13(4):1503402
Li F, Du M, Zheng Q (2016) Dopamine/silica nanoparticle assembled, microscale porous structure for versatile superamphiphobic coating. ACS Nano 10(2):2910–2921
Koch K, Bhushan B, Jung YC, Barthlott W (2009) Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 5(7):1386–1393
Ma M, Mao Y, Gupta M, Gleason KK, Rutledge GC (2005) Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 38(23):9742–9748
Lu X, Wang C, Wei Y (2009) One-dimensional composite nanomaterials: synthesis by electrospinning and their applications. Small 5(21):2349–2370
Wu L, Li L, Li B, Zhang J, Wang A (2015) Magnetic, durable, and superhydrophobic polyurethane@Fe3O4@SiO2@fluoropolymer sponges for selective oil absorption and oil/water separation. ACS Appl Mater Interfaces 7(8):4936–4946
Pereira C, Alves C, Monteiro A et al (2011) Designing novel hybrid materials by one-pot co-condensation: from hydrophobic mesoporous silica nanoparticles to superamphiphobic cotton textiles. ACS Appl Mater Interfaces 3(7):2289–2299
Li F, Du M, Zheng Z, Song Y, Zheng Q (2015) A facile, multifunctional, transparent, and superhydrophobic coating based on a nanoscale porous structure spontaneously assembled from branched silica nanoparticles. Adv Mater Interfaces 2(13):1500201
Zhu T, Cai C, Guo J, Wang R, Zhao N, Xu J (2017) Ultra water repellent polypropylene surfaces with tunable water adhesion. ACS Appl Mater Interfaces 9(11):10224–10232
Xie Q, Xu J, Feng L et al (2004) Facile creation of a super-amphiphobic coating surface with bionic microstructure. Adv Mater 16(4):302–305
Osborne JH (2001) Observations on chromate conversion coatings from a sol–gel perspective. Prog Org Coat 41(4):280–286
Poovarodom S, Hosseinpour D, Berg JC (2008) Effect of particle aggregation on the mechanical properties of a reinforced organic–inorganic hybrid sol–gel composite. Ind Eng Chem Res 47(8):2623–2629
Simpson JT, Hunter SR, Aytug T (2015) Superhydrophobic materials and coatings: a review. Rep Prog Phys 78(8):086501
Bayer I (2017) On the durability and wear resistance of transparent superhydrophobic coatings. Coatings 7(12):12
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26(1):62–69
Diré S, Babonneau F, Sanchez C, Livage J (1992) Sol–gel synthesis of siloxane oxide hybrid coatings [Si(CH3)2O·MOX–M=Si, Ti, Zr, Al] with luminescent properties. J Mater Chem 2(2):239–244
Crivello JV, Mao Z (1997) Synthesis of novel multifunctional siloxane oligomers using sol–gel techniques and their photoinitiated cationic polymerization. Chem Mater 9(7):1554–1561
Buckley AM, Greenblatt M (1994) The sol–gel preparation of silica gels. J Chem Edu 71(7):599–602
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 11378 kb)
Supplementary material 2 (MP4 9805 kb)
Supplementary material 3 (MP4 16144 kb)
Supplementary material 4 (MP4 12004 kb)
Supplementary material 5 (MP4 11837 kb)
Rights and permissions
About this article
Cite this article
Chen, X., Chen, Y., Jin, T. et al. Fabrication of superhydrophobic coating from non-fluorine siloxanes via a one-pot sol–gel method. J Mater Sci 53, 11253–11264 (2018). https://doi.org/10.1007/s10853-018-2348-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2348-7