Abstract
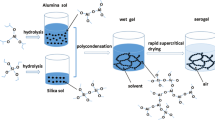
Alumina aerogel is of great interest for many potential applications because of high surface areas, intrinsic acid, and excellent mechanical properties. Different particle and pore size distribution can show superior performance in some applications, and therefore, the realization of the regulation of alumina particle and pore size has important industrialization significance. Herein, we demonstrate a simple method to prepare alumina aerogels with high surface areas and tunable pore size using sol–gel method with adding polyethylene glycol (PEG). And PEG is used as not only complexing agent to change the size of alumina aerogel particles and enhance the three-dimensional interconnected network structure, but also phase separation agent to promote the formation of macroporous. By changing the molecular mass of PEG and the amount of PEG of the same molecular mass, tunable pore size can be easily achieved. Owing to their high surface area and the three-dimensional network structure for promoting the transport of material into mesoporous where reactions take place, Al2O3 with centered pore size distribution was demonstrated to have excellent catalytic performance. In particular, the prepared Al2O3 adding 0.03 molar ratio of PEG-8000 exhibits the excellent catalytic activity for formaldehyde.











Similar content being viewed by others
References
Suh DJ, Park TJ (1996) Sol-gel strategies for pore size control of high-surface-area transition-metal oxide aerogels. Chem Mater 8:509–513
McGrath KM, Dabbs DM, Yao N et al (1997) Formation of a silicate L-3 phase with continuously adjustable pore sizes. Science 277:552–556
Meng X, Duan L, Qin H et al (2014) A novel synthesis and characterization of ordered meso/macroporous alumina with hierarchical and adjustable pore size. J Nanosci Nanotechnol 14:7340–7344
Yoo J, Bang Y, Han SJ et al (2016) Hydrogen production by steam reforming of liquefied natural gas (LNG) over magnesium-doped nickel-alumina aerogel catalyst. J Nanosci Nanotechnol 16:10835–10840
Zhao X, Cao Y, Li H et al (2017) Sc promoted and aerogel confined Ni catalysts for coking-resistant dry reforming of methane. RSC Adv 7:4735–4745
Yoo J, Bang Y, Han SJ et al (2015) Hydrogen production by tri-reforming of methane over nickel–alumina aerogel catalyst. J Mol Catal A-Chem 410:74–80
Zhao X, Lu M, Li H et al (2017) In situ preparation of Ni nanoparticles in cerium-modified silica aerogels for coking- and sintering-resistant dry reforming of methane. New J Chem 41:4869–4878
Cao Y, Lu M, Fang J et al (2017) Hexagonal boron nitride supported mesoSiO2-confined Ni catalysts for dry reforming of methane. Chem Commun 53:7549–7552
Wang W, Zhang K, Yang Y et al (2014) Synthesis of mesoporous Al2O3 with large surface area and large pore diameter by improved precipitation method. Micropor Mesopor Mater 193:47–53
Kelly DN, Wakabayashi RH, Stacy AM et al (2014) A modified sol–gel technique for pore size control in porous aluminum oxide nanowire templates. ACS Appl Mater Interfaces 6:20122–20129
Rouquerol J, Avnir D, Fairbridge CW et al (1994) Recommendations for the characterization of porous solids. Pure Appl Chem 66:1739–1758
Brinker CJ, Lu YF, Sellinger A et al (1999) Evaporation-induced self-assembly: nanostructures made easy. Adv Mater 11:579–585
Yuan Q, Yin A, Luo C et al (2008) Facile synthesis for ordered mesoporous gamma-aluminas with high thermal stability. J Am Chem Soc 130:3465–3472
Bagshaw SA, Prouzet E, Pinnavaia TJ (1995) Templating of mesoporous molecular-sieves by nonionic polyethylene oxide surfactants. Science 269:1242–1244
Tian BZ, Liu XY, Tu B et al (2003) Self-adjusted synthesis of ordered stable mesoporous minerals by acid–base pairs. Nat Mater 2:159–163
Baumann TF, Gash AE, Chinn SC et al (2005) Synthesis of high-surface-area alumina aerogels without the use of alkoxide precursors. Chem Mater 17:395–401
Wu Q, Zhang F, Yang J et al (2011) Synthesis of ordered mesoporous alumina with large pore sizes and hierarchical structure. Micropor Mesopor Mater 143:406–412
Yao N, Xiong GX, Zhang YH et al (2001) Preparation of novel uniform mesoporous alumina catalysts by the sol–gel method. Catal Today 68:97–109
Yabuki M, Takahashi R, Sato S et al (2002) Silica alumina catalysts prepared in sol–gel process of TEOS with organic additives. Phys Chem 4:4830–4837
Drisko GL, Zelcer A, Luca V et al (2010) One-pot synthesis of hierarchically structured ceramic monoliths with adjustable porosity. Chem Mater 22:4379–4385
Lafon O, Thankamony ASL, Kobayashi T et al (2013) Mesoporous silica nanoparticles loaded with surfactant: low temperature magic angle spinning C-13 and Si-29 NMR enhanced by dynamic nuclear polarization. J Phys Chem C 117:1375–1382
Hartmann S, Sachse A, Galarneau A (2012) Challenges and strategies in the synthesis of mesoporous alumina powders and hierarchical alumina monoliths. Materials 5:336–349
Tokudome Y, Fujita K, Nakanishi K et al (2007) Synthesis of monolithic Al2O3 with well-defined macropores and mesostructured skeletons via the sol–gel process accompanied by phase separation. Chem Mater 19:3393–3398
Li L, Duan W, Yuan Q et al (2009) Hierarchical gamma-Al2O3 monoliths with highly ordered 2D hexagonal mesopores in macroporous walls. Chem Commun (41):6174–6176
Dhainaut J, Deville S, Amirouche I et al (2016) A reliable method for the preparation of multiporous alumina monoliths by ice-templating. Inorganics 4(1):6
Dhainaut J, Piana G, Deville S et al (2014) Freezing-induced ordering of block copolymer micelles. Chem Commun 50:12572–12574
Ewlad-Ahmed AM, Morris MA, Patwardhan SV et al (2012) Removal of formaldehyde from air using functionalized silica supports. Environ Sci Technol 46:13354–13360
Zhang L, Yu X, Hu H et al (2015) Facile synthesis of iron oxides/reduced graphene oxide composites: application for electromagnetic wave absorption at high temperature. Sci Rep 5:9298
Chen B, Zhu X, Wang Y et al (2016) Gold stabilized on various oxide supports catalyzing formaldehyde oxidation at room temperature. Chin J Catal 37:1729–1737
Yan Z, Xu Z, Yu J et al (2016) Enhanced formaldehyde oxidation on CeO2/AlOOH-supported Pt catalyst at room temperature. Appl Catal B-Environ 199:458–465
Han Y, Zheng Z, Yin C et al (2016) Catalytic oxidation of formaldehyde on iron ore tailing. J Taiwan Inst Chem Eng 66:217–221
Bai B, Qiao Q, Li J et al (2016) Synthesis of three-dimensional ordered mesoporous MnO2 and its catalytic performance in formaldehyde oxidation. Chin J Catal 37:27–31
Chen B, Zhu X, Crocker M et al (2014) FeOx-supported gold catalysts for catalytic removal of formaldehyde at room temperature. Appl Catal B-Environ 154:73–81
Gheorghiu S, Coppens MO (2004) Optimal bimodal pore networks for heterogeneous catalysis. AIChE J 50:812–820
Groen JC, Zhu W, Brouwer S et al (2007) Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication. J Am Chem Soc 129:355–360
Yuan ZY, Ren TZ, Azioune A et al (2006) Self-assembly of hierarchically mesoporous-macroporous phosphated nanocrystalline aluminum (oxyhydr)oxide materials. Chem Mater 18:1753–1767
Dacquin J, Dhainaut J, Duprez D et al (2009) An efficient route to highly organized tunable macroporous-mesoporous alumina. J Am Chem Soc 131:12896
Muhler M, Schütze J, Wesemann M et al (1990) The nature of the iron oxide-based catalyst for dehydrogenation of ethylbenzene to styrene: I. Solid-state chemistry and bulk characterization. J Catal 126:339–360
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+, and Fe3+, ions in oxide materials. Appl Surf Sci 254:2441–2449
Xue X, Hanna K, Deng N (2009) Fenton-like oxidation of Rhodamine B in the presence of two types of iron (II, III) oxide. J Hazard Mater 166:407–414
Usman M, Faure P, Ruby C (2012) Remediation of PAH-contaminated soils by magnetite catalyzed fenton-like oxidation. Appl Catal B Environ 117:10–17
Rusevova K, Kopinke FD, Georgi A (2012) Nano-sized magnetic iron oxides as catalysts for heterogeneous fenton-like reactions-influence of Fe(II)/Fe(III) ratio on catalytic performance. J Hazard Mater 241–242:433–440
Acknowledgements
We gratefully acknowledge the financial support of the Nature Science Foundation of Jiangsu Province of China (BK20151408) and the Fundamental Research Funds for the Central Universities (3207046418, KYLX16_0193, and 2242017k1G006).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors.
Corresponding author
Ethics declarations
Conflict of interest
The author declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mei, J., Shao, Y., Lu, S. et al. Synthesis of Al2O3 with tunable pore size for efficient formaldehyde oxidation degradation performance. J Mater Sci 53, 3375–3387 (2018). https://doi.org/10.1007/s10853-017-1795-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1795-x