Abstract
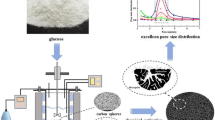
In the hydrothermal carbonization of carbohydrates, such as sucrose as raw material in this study, activated carbon microspheres were synthesized by two steps of hydrothermal carbonization (180 °C) and further heat treatment in nitrogen (1000 °C). The main purpose of this study was to investigate the effects of additives, such as H3PO4, ZnCl2, SnCl2, and CaCl2, on the surface characteristics and toluene adsorption ability by adding them into the two processes. The structural, chemical, and adsorption properties of sucrose-derived activated carbon microspheres were characterized using nitrogen adsorption, scanning electron microscopy, X-ray diffraction, Fourier transform infrared spectroscopy, water contact angles, and dynamic adsorption of toluene. Results showed that additives played important roles in the synthesis process. The addition of CaCl2 in the hydrothermal process, the specific surface area of activated carbon spheres increased up to 1180 m2 g−1 compared with that of the blank sample (i.e., 724 m2 g−1). By contrast, the addition of H3PO4 in the heat treatment process increased the specific surface area to 1529 m2 g−1. Moreover, the micromorphology of activated carbon microspheres was more homogeneous when additives were added in the heat treatment process, but the activated carbon microspheres were more hydrophobic when additives were added in the hydrothermal process. These findings may help researchers to understand the influence of additives on the preparation of hydrochar-derived activated carbon.








Similar content being viewed by others
References
Jain A, Balasubramanian R, Srinivasan MP (2016) Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chem Eng J 283:789–805
Donar YO, Çağlar E, Sınağ A (2016) Preparation and characterization of agricultural waste biomass based hydrochars. Fuel 183:366–372
Lynam JG, Toufiq Reza M, Vasquez VR, Coronella CJ (2012) Effect of salt addition on hydrothermal carbonization of lignocellulosic biomass. Fuel 99:271–273
Chen JZ, Chen ZH, Wang CH, Li XD (2012) Calcium-assisted hydrothermal carbonization of an alginate for the production of carbon microspheres with unique surface nanopores. Mater Lett 67:365–368
Reiche S, Kowalew N, Schlögl R (2015) Influence of synthesis pH and oxidative strength of the catalyzing acid on the morphology and chemical structure of hydrothermal carbon. ChemPhysChem 16:579–587
Supriya BS, Nagaraja P, Byrappa K (2015) Hydrothermal synthesis and characterization of carbon spheres using citric-acid-catalyzed carbonization of starch. e-Polymers 15:179–183
Román S, Valente Nabais JM, Ledesma B, González JF, Laginhas C, Titirici MM (2013) Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater 165:127–133
Pang LY, Zou B, Zou YC, Han X, Cao LY, Wang W, Guo YP (2016) A new route for the fabrication of corn starch-based porous carbon as electrochemical supercapacitor electrode material. Colloids Surf A 504:26–33
Lynam JG, Coronella CJ, Yan W, Reza MT, Vasquez VR (2011) Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour Technol 102:6192–6199
Sevilla M, Fuertes AB (2009) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47:2281–2289
Falco C, Baccile N, Titirici MM (2011) Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem 13:3273–3281
Titirici MM, Thomas A, Yu SH, Müller J, Antonietti M (2007) A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chem Mater 19:4205–4212
Kang SM, Li XL, Fan J, Chang J (2012) Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind Eng Chem Res 51:9023–9031
Wang LL, Guo YP, Zou B et al (2011) High surface area porous carbons prepared from hydrochars by phosphoric acid activation. Bioresour Technol 102:1947–1950
Zhu XD, Liu YC, Zhou C, Luo G, Zhang SC, Chen JM (2014) A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline. Carbon 77:627–636
Zhao Y, Li W, Zhao X, Wang DP, Liu SX (2013) Carbon spheres obtained via citric acid catalysed hydrothermal carbonisation of cellulose. Mater Res Innov 17:546–551
Cai HM, Lin XY, Tian LY, Luo XG (2016) One-step hydrothermal synthesis of carbonaceous spheres from glucose with an aluminum chloride catalyst and its adsorption characteristic for uranium(VI). Ind Eng Chem Res 55:9648–9656
Takeuchi Y, Jin FM, Tohji K, Enomoto H (2008) Acid catalytic hydrothermal conversion of carbohydrate biomass into useful substances. J Mater Sci 43:2472–2475. doi:10.1007/s10853-007-2021-z
Qi XH, Li LY, Tan TF, Chen WT, Smith RL Jr (2013) Adsorption of 1-butyl-3-methylimidazolium chloride ionic liquid by functional carbon microspheres from hydrothermal carbonization of cellulose. Environ Sci Technol 47:2792–2798
Fechler N, Wohlgemuth SA, Jäker P, Antonietti M (2013) Salt and sugar: direct synthesis of high surface area carbon materials at low temperatures via hydrothermal carbonization of glucose under hypersaline conditions. J Mater Chem A 1:9418–9421
Quesada-Plata F, Ruiz-Rosas R, Morallón E, Cazorla-Amorós D (2016) Activated carbons prepared through H3PO4-assisted hydrothermal carbonisation from biomass wastes: porous texture and electrochemical performance. ChemPlusChem 81:1349–1359
Liang JL, Liu YH, Zhang J (2011) Effect of solution pH on the carbon microsphere synthesized by hydrothermal carbonization. Procedia Environ Sci 11:1322–1327
Jain A, Aravindan V, Jayaraman S et al (2013) Activated carbons derived from coconut shells as high energy density cathode material for Li-ion capacitors. Sci Rep UK 3:3002
Romero-Anaya AJ, Ouzzine M, Lillo-Ródenas M, Linares-Solano A (2014) Spherical carbons: synthesis, characterization and activation processes. Carbon 68:296–307
Jain A, Balasubramanian R, Srinivasan MP (2015) Tuning hydrochar properties for enhanced mesopore development in activated carbon by hydrothermal carbonization. Microporous Mesoporous Mater 203:178–185
Jain A, Jayaraman S, Balasubramanian R, Srinivasan MP (2014) Hydrothermal pre-treatment for mesoporous carbon synthesis: enhancement of chemical activation. J Mater Chem A 2:520–528
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem A Eur J 15:4195–4203
Yao CH, Shin YS, Wang LQ et al (2007) Hydrothermal dehydration of aqueous fructose solutions in a closed system. J Phys Chem C 111:15141–15145
Aydıncak K, Tr Yumak, Sınağ A, Esen B (2012) Synthesis and characterization of carbonaceous materials from saccharides (glucose and lactose) and two waste biomasses by hydrothermal carbonization. Ind Eng Chem Res 51:9145–9152
Wang Q, Li H, Chen LQ, Huang XJ (2001) Monodispersed hard carbon spherules with uniform nanopores. Carbon 39:2211–2214
Sevilla M, Lota G, Fuertes AB (2007) Saccharide-based graphitic carbon nanocoils as supports for PtRu nanoparticles for methanol electrooxidation. J Power Sources 171:546–551
Mer VKL (1952) Nucleation in phase transitions. Ind Eng Chem 44:1270–1277
Zhang CY, Lin S, Peng JJ, Hong YZ, Wang ZY, Jin XB (2017) Preparation of highly porous carbon through activation of NH4Cl induced hydrothermal microsphere derivation of glucose. RSC Adv 7:6486–6491
Hao SW, Hsu CH, Liu YG, Chang BK (2016) Activated carbon derived from hydrothermal treatment of sucrose and its air filtration application. RSC Adv 6:109950–109959
Titirici MM, White RJ, Falco C, Sevilla M (2012) Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ Sci 5:6796–6822
Zhang PF, Zhang ZY, Chen JH, Dai S (2015) Ultrahigh surface area carbon from carbonated beverages: combining self-templating process and in situ activation. Carbon 93:39–47
Li M, Cullen DA, Sasaki K, Marinkovic NS, More K, Adzic RR (2013) Ternary electrocatalysts for oxidizing ethanol to carbon dioxide: making ir capable of splitting C–C bond. J Am Chem Soc 135:132–141
Zhang W, Qu Z, Li X, Wang Y, Ma D, Wu J (2012) Comparison of dynamic adsorption/desorption characteristics of toluene on different porous materials. J Environ Sci China 24:520–528
Lu HF, Cao JJ, Zhou Y, Zhan DL, Chen YF (2013) Novel hydrophobic PDVB/R-SiO2 for adsorption of volatile organic compounds from highly humid gas stream. J Hazard Mater 262:83–90
Acknowledgements
We would like to acknowledge the financial support provided by the Natural Science Foundation of China (No. 21506194, 21676255) and Zhejiang Provincial Natural Science Foundation of China (No. Y14E080008, Y16B070025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, H., Lu, X., Wang, Y. et al. Effects of additives on sucrose-derived activated carbon microspheres synthesized by hydrothermal carbonization. J Mater Sci 52, 10787–10799 (2017). https://doi.org/10.1007/s10853-017-1258-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1258-4