Abstract
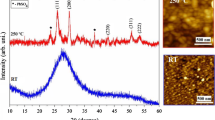
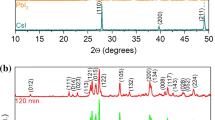
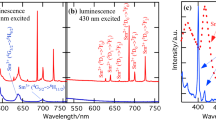
A mechanically induced solid-state reaction method for the synthesis of organic–inorganic hybrid perovskites, such as methylammonium lead iodide (CH3NH3PbI3) is reported. The perovskites were synthesized both in bulk and Al2O3-supported forms. The phase- and structural-formation mechanisms of such perovskites are also investigated. The experiments suggest that diffusion of PbI2 into CH3NH3I crystals is a rate-limiting step of the reaction process. It is also shown that water (humidity) significantly influences the reaction kinetics. UV–Vis–NIR absorption and photoluminescence spectroscopies indicate that the band edge and emission characteristics of the as-fabricated materials strongly depend on their particle size.






Similar content being viewed by others
References
Burschka J, Pellet N, Moon S-J et al (2013) Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499:316–319. doi:10.1038/nature12340
Jeon NJ, Noh JH, Kim YC et al (2014) Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat Mater 13:1–7. doi:10.1038/nmat4014
Lee MM, Teuscher J, Miyasaka T et al (2012) Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338:643–647. doi:10.1126/science.1228604
Ball JM, Lee MM, Hey A, Snaith HJ (2013) Low-temperature processed meso-superstructured to thin-film perovskite solar cells. Energy Environ Sci 6:1739. doi:10.1039/c3ee40810h
Xiao M, Huang F, Huang W et al (2014) A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew Chem Int Ed 3169:1–7. doi:10.1002/anie.201405334
Ragoussi M-E, Torres T (2015) New generation solar cells: concepts, trends and perspectives. Chem Commun 51:3957–3972. doi:10.1039/c4cc09888a
Baikie T, Fang Y, Kadro JM et al (2013) Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J Mater Chem A 1:5628. doi:10.1039/c3ta10518k
Kojima A, Teshima K, Shirai Y, Miyasaka T (2009) Organo metal halide perovskites as visible-light sensitizer for photovoltaic cells. J Am Chem Soc 131:6050–6051. doi:10.1021/Ja809598r
Savenije TJ, Ponseca CS, Kunneman L et al (2014) Thermally activated exciton dissociation and recombination control the carrier dynamics in organometal halide perovskite. J Phys Chem Lett 5:2189–2194. doi:10.1021/jz500858a
Stranks SD, Eperon GE, Grancini G et al (2013) Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 342:341–344. doi:10.1126/science.1243982
Dong Q, Fang Y, Shao Y et al (2015) Electron-hole diffusion lengths > 175 m m in solution-grown CH3NH3PbI3 single crystals. Science 347:967–970
Yang W, Nog J, Jeon N et al (2015) High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348:1234–1237
Stranks SD, Nayak PK, Zhang W et al (2015) Formation of thin films of organic-inorganic perovskites for high-efficiency solar cells. Angew Chem Int Ed 54:3240–3248. doi:10.1002/anie.201410214
Yantara N, Sabba D, Fang Y et al (2015) Loading of mesosporous titania films by CH3NH3PbI3 perovskite, single step vs sequential deposition. Chem Commun 51:4603–4606. doi:10.1039/C4CC09556A
Liu M, Johnston MB, Snaith HJ (2013) Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501:395–398. doi:10.1038/nature12509
Manser JS, Kamat PV (2014) Band filling with free charge carriers in organometal halide perovskites. Nat Photonics 8:737–743. doi:10.1038/nphoton.2014.171
Wong AB, Lai M, Eaton SW et al (2015) Growth and anion exchange conversion of CH3NH3PbX3 nanorod arrays for light-emitting diodes. Nano Lett 15:5519–5524. doi:10.1021/acs.nanolett.5b02082
Xing J, Liu XF, Zhang Q et al (2015) Vapor phase synthesis of organometal halide perovskite nanowires for tunable room-temperature nanolasers. Nano Lett 15:4571–4577. doi:10.1021/acs.nanolett.5b01166
Im J, Luo J, Franckevicius M et al (2015) Nanowire perovskite solar cell. Nano Lett. doi:10.1021/acs.nanolett.5b00046
Saparov B, Mitzi DB (2016) Organic–inorganic perovskites: structural versatility for functional materials design. Chem Rev. 116:4558. doi:10.1021/acs.chemrev.5b00715
Chen Y-S, Manser JS, Kamat PV (2014) All solution-processed lead halide perovskite-BiVO4 tandem assembly for photolytic solar fuels production. J Am Chem Soc 137:974–981. doi:10.1021/ja511739y
Zhu H, Fu Y, Meng F et al (2015) Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat Mater 14:636–643. doi:10.1038/nmat4271
Gil-Escrig L, Longo G, Pertegás A et al (2015) Efficient photovoltaic and electroluminescent perovskite devices. Chem Commun 51:569–571. doi:10.1039/c4cc07518h
Fang Y, Dong Q, Shao Y et al (2015) Highly narrowband perovskite single-crystal photodetectors enabled by surface-charge recombination. Nat Photonics 9:679–686. doi:10.1038/nphoton.2015.156
Yakunin S, Sytnyk M, Kriegner D et al (2015) Detection of X-ray photons by solution-processed lead halide perovskites. Nat Photonics 9:444–449. doi:10.1038/Nphoton.2015.82
Guo Y, Liu C, Tanaka H, Nakamura E (2015) Air-stable and solution-processable perovskite photodetectors for solar-blind UV and visible light. J Phys Chem Lett 6:535–539. doi:10.1021/jz502717g
Ha S, Shen C, Zhang J, Xiong Q (2015) Laser cooling of organic-inorganic lead halide perovskites. Nat Photonics 10:115–121. doi:10.1038/nphoton.2015.243
Dimesso L, Dimamay M, Hamburger M, Jaegermann W (2014) Properties of CH3NH3 PbX3 (X=I, Br, Cl) powders as precursors for organic/inorganic solar cells. Chem Mater 26:6762–6770
Im J-H, Lee C-R, Lee J-W et al (2011) 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 3:4088. doi:10.1039/c1nr10867k
Thoai DT, Hu Y, Koch S (1990) Influence of the confinement potential on the electron-hole-pair states in semiconductor microcrystallites. Phys Rev B 42:261–266. doi:10.1103/PhysRevB.42.11261
Hirasawa M, Ishihara T, Goto T et al (1994) Magnetoabsorption of the lowest exciton in perovskite-type compound (CH3NH3)PbI3. Phys B Condens Matter 201:427–430. doi:10.1016/0921-4526(94)91130-4
Suryanarayana C (2001) Mechanical alloying and milling. Prog Mater Sci 46:1–184. doi:10.1016/S0079-6425(99)00010-9
Takacs L (2002) Self-sustaining reactions induced by ball milling. Prog Mater Sci 47:355–414. doi:10.1016/S0079-6425(01)00002-0
Urakaev FKh, Boldyrev VV (2000) Mechanism and kinetics of mechanochemical processes in comminuting devices 1. Theory. Powder Technol 107:93–107. doi:10.1533/9781845699444.1.9
Manukyan KV, Mason BA, Groven LJ et al (2012) Tailored reactivity of Ni + Al nanocomposites: microstructural correlations. J Phys Chem C 116:21027–21038. doi:10.1021/jp303407e
Beldon PJ, Fábián L, Stein RS et al (2010) Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew Chem Int Ed 49:9640–9643. doi:10.1002/anie.201005547
Adams CJ, Haddow MF, Lusi M, Orpen AG (2010) Crystal engineering of lattice metrics of perhalometallate salts and MOFs. Proc Natl Acad Sci U S A 107:16033–16038. doi:10.1073/pnas.0910146107
Friščić T, Halasz I, Beldon PJ et al (2013) Real-time and in situ monitoring of mechanochemical milling reactions. Nat Chem 5:66–73. doi:10.1038/nchem.1505
Kaupp G (2003) Solid-state molecular syntheses: complete reactions without auxiliaries based on the new solid-state mechanism. CrystEngComm 5:117–133. doi:10.1039/b303432a
Wang G (2013) Mechanochemical organic synthesis. Chem Soc Rev 42:7668–7700. doi:10.1039/c3cs35526h
James SL, Adams CJ, Bolm C et al (2012) Mechanochemistry: opportunities for new and cleaner synthesis. Chem Soc Rev 41:413–447. doi:10.1039/c1cs15171a
André V, Hardeman A, Halasz I et al (2011) Mechanosynthesis of the metallodrug bismuth subsalicylate from Bi2O3 and structure of bismuth salicylate without auxiliary organic ligands. Angew Chem Int Ed 50:7858–7861. doi:10.1002/anie.201103171
Delori A, Friscic T, Jones W et al (2012) The role of mechanochemistry and supramolecular design in the development of pharmaceutical materials. CrystEngComm 14:2350–2362. doi:10.1039/C2CE06582G
Manukyan KV, Lin YC, Rouvimov S et al (2013) Microstructure-reactivity relationship of Ti + C reactive nanomaterials. J Appl Phys. doi:10.1063/1.4773475
Acknowledgements
The authors gratefully acknowledge the financial support of the Ministry of Education and Science of the Russian Federation in the framework of Increase Competitiveness Program of NUST “MISiS” (#K3-2015-016). This work was also partially supported by the Ministry of Education and Science and Education of the Russian Federation in the framework of Increase Competitiveness Program of NUST “MISIS” Grant # K2-2014-001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manukyan, K.V., Yeghishyan, A.V., Moskovskikh, D.O. et al. Mechanochemical synthesis of methylammonium lead iodide perovskite. J Mater Sci 51, 9123–9130 (2016). https://doi.org/10.1007/s10853-016-0165-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0165-4