Abstract
The thermodynamics and kinetics fundaments of grain growth in binary substitutional alloys were analyzed using the thermodynamic extremal principle. Applying the regular solution approximation, a new equation for solute segregation at steady-state diffusion is proposed, which suggests reduced solute segregation as the grain boundary (GB) solute concentration increases, differently from previous models [Acta Mater 2009;57(5):1466, Acta Mater 2012;60:4833, Scripta Mater 2010;63:989] that adopt constant segregation enthalpy. Furthermore, a self-consistent consideration has been carried out to account for the coupled changes in GB energy and GB mobility as a result of solute segregation. On this basis, the quantitative relation is evaluated between the thermodynamic and kinetic effects of solute segregation to determine the dominant role in retarding and even suppressing grain growth, by comparison of the dimensionless GB energy (i.e., the GB energy of alloy over that of pure solvent) and the dimensionless effective GB mobility (i.e., the effective GB mobility over that of pure solvent): the kinetic effect prevails if the dimensionless effective GB mobility is smaller than the dimensionless GB energy, and vice versa. The present model is adopted to describe well the experimental results for Fe–P alloys, and nanocrystalline Ni–P and Pd–Zr alloys.






Similar content being viewed by others
Notes
The value of diffusion coefficient of P in the grain boundaries of chemical similar Fe [55] is found within \( s\delta D_{\text{P}}^{\text{GB}} \) = 3.30 × 10−15exp(−92.47 × 103/R g T) at 950–1139 K [s as the segregation factor approximately evaluated by exp(−ΔH seg/R g T)]. The value of s is thus given as 207, the mean of 116–298 utilizing s = exp(−ΔH seg/R g T) with ΔH seg = −45 kJ mol−1 [13] at T = 1139–950 K. Therefore, the value of \( D_{\text{P}}^{\text{GB}} \) is calculated to be 3.5 × 10−16 m2 s−1 at T = 623 K.
References
Krill CE, Ehrhardt H, Birringer R (2005) Thermodynamic stabilization of nanocrystallinity. Z Metallkunde 96:1134–1141
Detor AJ, Miller MK, Schuh CA (2006) Solute distribution in nanocrystalline Ni-W alloys examined through atom probe tomography. Philos Mag 86:4459–4475
Detor AJ, Miller MK, Schuh CA (2007) Measuring grain-boundary segregation in nanocrystalline alloys: direct validation of statistical techniques using atom probe Tomography. Philos Mag Lett 87:581–587
Detor AJ, Schuh CA (2007) Tailoring and patterning the grain size of nanocrystalline alloys. Acta Mater 55:371–379
Darling KA, Chan RN, Wong PZ, Semones JE, Scattergood RO, Koch CC (2008) Grain-size stabilization in nanocrystalline FeZr alloys. Scripta Mater 59:530–533
Koch CC, Scattergood RO, Darling KA, Semones JE (2008) Stabilization of nanocrystalline grain sizes by solute additions. J Mater Sci 43:7264–7272. doi:10.1007/s10853-008-2870-0
Darling KA, VanLeeuwen BK, Semones JE, Koch CC, Scattergood RO, Kecskes LJ, Mathaudhu SN (2011) Stabilized nanocrystalline iron-based alloys: Guiding efforts in alloy selection. Mater Sci Eng A 528:4365–4371
Chen YZ, Herz A, Kirchheim R (2011) Grain boundary segregation of carbon and formation of nanocrystalline iron-carbon alloys by ball milling. Mater Sci Forum 667–669:265–270
Liu F (2005) Precipitation of a metastable Fe(Ag) solid solution upon annealing of supersaturated Fe(Ag) thin film prepared by pulsed laser deposition. Appl Phys A 81:1095–1098
Liu KW, Mücklich F (2001) Thermal stability of nano-RuAl produced by mechanical alloying. Acta Mater 49:395–403
Natter H, Löffler MS, Krill CE, Hempelmann R (2001) Crystallite growth of nanocrystalline transition metals studied in situ by high temperature synchrotron X-ray diffraction. Scripta Mater 44:2321–2325
Weissmüller J (1993) Alloy effects in nanostructures. Nanostruct Mater 3:261–272
Kirchheim R (2002) Grain coarsening inhibited by solute segregation. Acta Mater 50:413–419
Kirchheim R (2007) Reducing grain boundary, dislocation line and vacancy formation energies by solute segregation. I. Acta Mater 55:5129–5138
Kirchheim R (2007) Reducing grain boundary, dislocation line and vacancy formation energies by solute segregation: II. Experimental evidence and consequences. Acta Mater 55:5139–5148
Liu F, Kirchheim R (2004) Nano-scale grain growth inhibited by reducing grain boundary energy through solute segregation. J Cryst Growth 264:385–391
Liu F, Kirchheim R (2004) Grain boundary saturation and grain growth. Scripta Mater 51:521–525
Liu F, Kirchheim R (2004) Comparison between kinetic and thermodynamic effects on grain growth. Thin Solid Films 466:108–113
Trelewicz JR, Schuh CA (2009) Grain boundary segregation and thermodynamically stable binary nanocrystalline alloys. Phys Rev B 79:094112
Detor AJ, Schuh CA (2007) Grain boundary segregation, chemical ordering and stability of nanocrystalline alloys: atomistic computer simulations in the Ni–W system. Acta Mater 55:4221–4232
Millett PC, Selvam RP, Bansal S, Saxena A (2005) Atomistic simulation of grain boundary energetics—effects of dopants. Acta Mater 53:3671–3678
Millett PC, Selvam RP, Saxena A (2006) Molecular dynamics simulations of grain size stabilization in nanocrystalline materials by addition of dopants. Acta Mater 54:297–303
Millett PC, Selvam RP, Saxena A (2007) Stabilizing nanocrystalline materials with dopants. Acta Mater 55:2329–2336
Chookajorn T, Murdoch HA, Schuh CA (2012) Design of stable nanocrystalline alloys. Science 337:951–954
Saber M, Kotan H, Koch C, Scattergood R (2013) Thermodynamic stabilization of nanocrystalline binary alloys. J Appl Phys 113:063515
Saber M, Kotan H, Koch C, Scattergood R (2013) A predictive model for thermodynamic stability of grain size in nanocrystalline ternary alloys. J Appl Phys 114:103510
Cahn JW (1962) The impurity-drag effect in grain boundary motion. Acta Metall 10:789–798
Lücke K, Stüwe HP (1971) On the theory of impurity controlled grain boundary motion. Acta Metall 19:1087–1099
Smith CS (1948) Grains, phases, and interphases: an interpretation of microstructure. Trans AIME 175:15–51
Burke JE (1949) Some factors affecting the rate of grain growth in metals. Trans Metall Soc AIME 175:73
Michels A, Krill CE, Ehrhardt H, Birringer R, Wu DT (1999) Modelling the influence of grain-size-dependent solute drag on the kinetics of grain growth in nanocrystalline materials. Acta Mater 47:2143–2152
Rabkin E (2000) On the grain size dependent solute and particle drag. Scripta Mater 42:1199–1206
Hillert M, Sundman B (1976) A treatment of the solute drag on moving grain boundaries and phase interfaces in binary alloys. Acta Metall 24:731–743
Svoboda J, Fischer FD, Gamsjäger E (2002) Influence of solute segregation and drag on properties of migrating interfaces. Acta Mater 50:967–977
Svoboda J, Fischer FD, Leindl M (2011) Transient solute drag in migrating grain boundaries. Acta Mater 59:6556–6562
Chen Z, Liu F, Wang HF, Yang W, Yang GC, Zhou YH (2009) Acta Mater 57:1466–1475
Chen Z, Liu F, Yang XQ, Shen CJ (2012) A thermokinetic description of nanoscale grain growth: analysis of the activation energy effect. Acta Mater 60:4833–4844
Gong MM, Liu F, Zhang K (2010) A thermokinetic description of nanoscale grain growth: analysis of initial grain boundary excess amount. Scripta Mater 63:989–992
Hillert M (2007) Phase equilibria, phase diagrams and phase transformations: their thermodynamic basis, 2nd edn. Cambridge University Press, New York
Svoboda J, Turek I, Fischer FD (2005) Application of the thermodynamic extremal principle to modeling of thermodynamic processes in material sciences. Philos Mag 85:3699–3707
Wang H, Liu F, Yang W, Chen Z, Yang G, Zhou Y (2008) Solute trapping model incorporating diffusive interface. Acta Mater 56:746–753
Hillert M (1965) On the theory of normal and abnormal grain growth. Acta Metall 13:227–238
Burke J, Turnbull D (1952) Recrystallization and grain growth. Prog Metal Phys 3:220
Plessis JD (1990) Surface Segregation. Trans Tech Pubn, Switzerland
Mclean D (1957) Grain boundaries in metals. Oxford University Press, Oxford
Lejček P, Hofmann S, Janovec J (2007) Prediction of enthalpy and entropy of solute segregation at individual grain boundaries of α-iron and ferrite steels. Mater Sci Eng A 462:76–85
Nishizawa T (2008) Thermodynamics of Microstructures. ASM International, OH
Hondros ED (1965) The influence of phosphorus in dilute solid solution on the absolute surface and grain boundary energies of iron. Proc R Soc 286:479–498
Takeuchi A, Inoue A (2000) Calculations of mixing enthalpy and mismatch entropy for ternary amorphous alloys. Mater Trans JIM 41:1372–1378
Färber B, Cadel E, Menand A, Schmitz G, Kirchheim R (2000) Phosphorus segregation in nanocrystalline Ni–3.6 at.% P alloy investigated with the tomographic atom probe (TAP). Acta Mater 48:789–796
Fowler RH, Guggenheim EA (1939) Statistical thermodynamics. Cambridge University Press, Cambridge
Vitos L, Ruban AV, Skriver HL, Kollár J (1998) The surface energy of metals. Surf Sci 411:186–202
Osmola D, Nolan P, Erb U, Palumbo G, Aust KT (1992) Microstructural evolution at large driving forces during grain growth of ultrafine-grained Ni-1.2wt% P. Phys Stat Sol A 131:569–575
Mishin Y, Herzig C, Bernardini J, Gust W (1997) Grain boundary diffusion: fundamentals to recent developments. Int Mater Rev 42:155–178
Mehrer H (1990) Diffusion in solid metals and alloys. Springer, Berlin
VanLeeuwen BK, Darling KA, Koch CC, Scattergood RO, Butler BG (2010) Thermal stability of nanocrystalline Pd81Zr19. Acta Mater 58:4292–4297
Acknowledgements
The authors are grateful to the financial support of National Basic Research Program of China (No. 2011CB610403), the Natural Science Foundation of China (Nos. 51134011 and 51431008), the Fundamental Research Fund of Northwestern Polytechnical University (No. JC20120223), and the China National Funds for Distinguished Young Scientists (No. 51125002). M.M. Gong is thanked for the financial support of the Doctorate Foundation of Northwestern Polytechnical University (CX201204). R. H. R. Castro is thanked for the financial support of the National Science Foundation (DMR 1055504).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
In Hillert’s derivation [39, 47], as the equilibrium state is corresponding to the minimum of total Gibbs energy in the system, the change of total Gibbs energy must remain zero when tiny solvent atoms with amount of dn A and solute atoms with amount of dn B are transferred from the bulk phase to the GB one, i.e., [47]
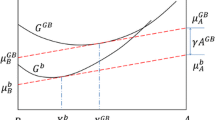
In general, the solution of Eq. (32) is \( \mu_{\text{A}}^{\text{GB}} = \mu_{\text{A}}^{\text{bulk}} \) and \( \mu_{\text{B}}^{\text{GB}} - \mu_{\text{B}}^{\text{bulk}} \) due to the arbitrary dn A and dn B. However, under the assumption of constant GB width yielding dn A + dn B = 0, the precondition of Eq. (32) is \( \mu_{\text{B}}^{\text{GB}} - \mu_{\text{B}}^{\text{bulk}} \) = \( \mu_{\text{B}}^{\text{GB}} - \mu_{\text{B}}^{\text{bulk}} \) identical to Eq. (15). That is to say, the steady-state diffusion of components implies the equilibrium between the bulk and GB phases.
Appendix 2
The change of total Gibbs free energy in the system can be given by,
According to Gibbs–Duhem relation, Eq. (33) is rewritten as,
Then, with mass conservation equations dn GBA + dn bulkA = 0 and dn GBB + dn bulkB = 0, the last equation is further simplified as,
Thereafter, combining Eq. (35) with Eq. (15) yields
With \( {\text{d}}n^{\text{GB}} \) = δdS/V m, Eq. (36) is changed into
Further incorporating Eq. (18) into Eq. (37) leads to
On this basis, the driving force P of grain growth can be presented according to its definition (i.e., P = dG/dV) as,
By means of S = 4πR 2/2 (1/2 implying a GB shared by two adjacent grains) and V = 4/3πR 3, Eq. (39) is thus simplified as,
Definitely, under steady-state diffusion, the GB energy can be regarded as the driving force of the grain growth affected by solute segregation.
Rights and permissions
About this article
Cite this article
Gong, M.M., Castro, R.H.R. & Liu, F. Modeling grain growth kinetics of binary substitutional alloys by the thermodynamic extremal principle. J Mater Sci 50, 4610–4621 (2015). https://doi.org/10.1007/s10853-015-9010-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9010-4