Abstract
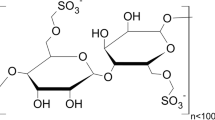
A range of anionic and cationic polycarboxylate ether (PCE) plasticizers with different molecular architectures (molecular weights, side chain lengths, and ratios of side chain density to backbone charge) are synthesized and tested to determine their effects on the rheological properties of fresh alkali-activated slag (AAS) pastes. A higher density of long side chains in the lower molecular weight polymers can provide a noticeable yield stress reduction, indicating a mild increase in workability compared to that of an unmodified AAS paste. It is hypothesized that side chains may have two important roles, i.e., providing steric hindrance to disperse particles after PCE adsorption on a particle surface, and also providing partial protection of the backbone charges against attachment of one PCE molecule to two or more slag particles, which is called bridging. This enhances the likelihood of adsorption on single particles, and thus increases the plasticizing action. A very similar plasticizing mechanism is observed for PCEs with similar structures but differing charge signs (cationic/anionic), which indicates that both anionic and cationic adsorption sites are available on AAS particle surfaces. The measured flow curves of all pastes are well described by the Herschel–Bulkley model with shear thinning behavior.















Similar content being viewed by others
References
Schneider M, Romer M, Tschudin M, Bolio H (2011) Sustainable cement production–present and future. Cem Concr Res 41:642–650
Provis JL, van Deventer JSJ (2014) Alkali-activated materials: state of the art report, RILEM TC 224-AAM. Springer, Dordrecht
van Deventer JSJ, Provis JL, Duxson P (2012) Technical and commercial progress in the adoption of geopolymer cement. Miner Eng 29:89–104
Bernal SA, Mejía de Gutierrez R, Pedraza AL, Provis JL, Rodríguez ED, Delvasto S (2011) Effect of binder content on the performance of alkali-activated slag concretes. Cem Concr Res 41:1–8
Kwan AKH, Fung WWS (2012) Roles of water film thickness and SP dosage in rheology and cohesiveness of mortar. Cem Concr Compos 34:121–130
Yamada K, Hanehara S (2003) Working mechanism of polycarboxylate superplasticizer considering the chemical structure and cement characteristics. In: 11th international congress on the chemistry of cement, Durban (CD-ROM Proceedings)
Ferrari L, Kaufmann J, Winnefeld F, Plank J (2010) Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J Colloid Interface Sci 347:15–24
Hanehara S, Yamada K (1999) Interaction between cement and chemical admixture from the point of cement hydration, absorption behaviour of admixture, and paste rheology. Cem Concr Res 29:1159–1165
Sakai E, Yamada K, Ota A (2003) Molecular structure and dispersion-adsorption mechanisms of comb-type superplasticizers used in Japan. J Adv Concr Technol 1:16–25
Yamada K (2011) Basics of analytical methods used for the investigation of interaction mechanism between cements and superplasticizers. Cem Concr Res 41:793–798
Plank J, Pollmann K, Zouaoui N, Andres PR, Schaefer C (2008) Synthesis and performance of methacrylic ester based polycarboxylate superplasticizers possessing hydroxy terminated poly(ethylene glycol) side chains. Cem Concr Res 38:1210–1216
Lesti M, Ng S, Plank J (2010) Ca2+-mediated interaction between microsilica and polycarboxylate comb polymers in a model cement pore solution. J Am Ceram Soc 93:3493–3498
Qiu XQ, Peng XY, Yi CH, Deng YH (2011) Effect of side chains and sulfonic groups on the performance of polycarboxylate-type superplasticizers in concentrated cement suspensions. J Dispers Sci Technol 32:203–212
Ran QP, Somasundaran P, Miao CW, Liu JP, Wu SS, Shen J (2009) Effect of the length of the side chains of comb-like copolymer dispersants on dispersion and rheological properties of concentrated cement suspensions. J Colloid Interface Sci 336:624–633
Zingg A, Winnefeld F, Holzer L, Pakusch J, Becker S, Gauckler L (2008) Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J Colloid Interface Sci 323:301–312
Marchon D, Sulser U, Eberhardt A, Flatt RJ (2013) Molecular design of comb-shaped polycarboxylate dispersants for environmentally friendly concrete. Soft Matter 9:10719–10728
Plank J, Sachsenhauser B (2006) Impact of molecular structure on zeta potential and adsorbed conformation of α-allyl-ω-methoxypolyethylene glycol–maleic anhydride superplasticizers. J Adv Concr Technol 4:233–239
Palacios M, Puertas F, Bowen P, Houst YF (2009) Effect of PCs superplasticizers on the rheological properties and hydration process of slag-blended cement pastes. J Mater Sci 44:2714–2723. doi:10.1007/s10853-009-3356-4
Criado M, Palomo A, Fernandez-Jimenez A, Banfill PFG (2009) Alkali activated fly ash: effect of admixtures on paste rheology. Rheol Acta 48:447–455
Habbaba A, Plank J (2010) Interaction between polycarboxylate superplasticizers and amorphous ground granulated blast furnace slag. J Am Ceram Soc 93:2857–2863
Plank J, Schrofl C, Gruber M, Lesti M, Sieber R (2009) Effectiveness of polycarboxylate superplasticizers in ultra-high strength concrete: the importance of PCE compatibility with silica fume. J Adv Concr Technol 7:5–12
Xu J, Tao L, Boyer C, Lowe AB, Davis TP (2010) Combining thio–bromo “click” chemistry and RAFT polymerization: a powerful tool for preparing functionalized multiblock and hyperbranched polymers. Macromolecules 43:20–24
Nguyen QD, Boger DV (1992) Measuring the flow properties of yield stress fluids. Annu Rev Fluid Mech 24:47–88
Johnson SB, Franks GV, Scales PJ, Boger DV, Healy TW (2000) Surface chemistry–rheology relationships in concentrated mineral suspensions. Int J Miner Proc 58:267–304
Dzuy NQ, Boger DV (1985) Direct yield stress measurement with the vane method. J Rheol 29:335–347
O’Brien RW, Jones A, Rowlands WN (2003) A new formula for the dynamic mobility in a concentrated colloid. Colloids Surf A 218:89–101
Gay C, Raphaël E (2001) Comb-like polymers inside nanoscale pores. Adv Colloid Interface Sci 94:229–236
Labbez C, Jonsson B, Pochard I, Nonat A, Cabane B (2006) Surface charge density and electrokinetic potential of highly charged minerals: experiments and Monte Carlo simulations on calcium silicate hydrate. J Phys Chem B 110:9219–9230
Song S, Jennings HM (1999) Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem Concr Res 29:159–170
Hanehara S, Yamada K (2008) Rheology and early age properties of cement systems. Cem Concr Res 38:175–195
Palacios M, Banfill PFG, Puertas F (2008) Rheology and setting of alkali-activated slag pastes and mortars: effect of organic admixture. ACI Mater J 105:140–148
Cyr M, Legrand C, Mouret M (2000) Study of the shear thickening effect of superplasticizers on the rheological behaviour of cement pastes containing or not mineral additives. Cem Concr Res 30:1477–1483
Jayasree C, Krishnan JM, Gettu R (2011) Influence of superplasticizer on the non-Newtonian characteristics of cement paste. Mater Struct 44:929–942
Feys D, Verhoeven R, De Schutter G (2009) Why is fresh self-compacting concrete shear thickening? Cem Concr Res 39:510–523
Grzeszczyk S, Janowska-Renkas E (2012) The influence of small particle on the fluidity of blast furnace slag cement paste containing superplasticizers. Constr Build Mater 26:411–415
Acknowledgements
This work has been funded through an Australian Research Council Linkage Project grant, cofunded by Zeobond Pty. Ltd., and also benefited from support through the Particulate Fluids Processing Centre, a Special Research Centre of the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kashani, A., Provis, J.L., Xu, J. et al. Effect of molecular architecture of polycarboxylate ethers on plasticizing performance in alkali-activated slag paste. J Mater Sci 49, 2761–2772 (2014). https://doi.org/10.1007/s10853-013-7979-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7979-0