Abstract
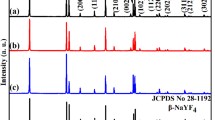
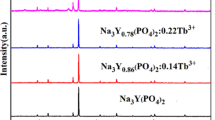
β-NaYF4:Ln3+ (Ln = Eu, Tb, Yb/Er, Yb/Tm) hexagonal microrods have been successfully synthesized through a facile molten salt method without any surfactant. X-ray diffraction, scanning electron microscopy (SEM), transmission electron microscopy, high-resolution transmission electron microscopy, and photoluminescence spectra were used to characterize the samples. It is found that at a preferred reaction temperature of 400 °C, the structure of β-NaYF4 can gradually transform from microtubes to microrods as reaction time extends from 0.5 to 4 h. Furthermore, as the molar ratio of NaF:RE3+ (RE represents the total amount of Y3+ and the doped rare earth elements such as Eu3+, Tb3+, Yb3+/Er3+, or Yb3+/Tm3+) increased, the phase of sample transforms from YF3 into NaYF4. Under the excitation of 395 nm ultraviolet light, β-NaYF4:5 %Eu3+ shows the emission lines of Eu3+ corresponding to 5D0-3 → 7F J (J = 1–4) transitions from 400 to 700 nm, resulting in red down-conversion (DC) light emission. When doped with 5 % Tb3+ ions, the strong DC fluorescence corresponding to 5D4 → 7F J (J = 6, 5, 4, 3) transitions with 5D4 → 7F J (green emission at 544 nm) being the most prominent group that has been observed. Moreover, upon 980 nm laser diode excitation, the Yb3+/Er3+- and Yb3+,Tm3+- co-doped β-NaYF4 samples exhibit bright yellow and blue upconversion (UC) luminescence, respectively, by two- or three-photon UC process. The luminescence mechanisms for the doped lanthanide ions were thoroughly analyzed.












Similar content being viewed by others
References
Kramer KW, Biner D, Frei G, Gudel HU, Hehlen MP, Luthi SR (2004) Chem Mater 16:1244
Zeng JH, Li ZH, Su J, Wang LY, Yan RO, Li YD (2006) Nanotechnology 17:3549
Yi GS, Lu HC, Zhao SY, Yue G, Yang WJ, Chen DP, Guo LH (2004) Nano Lett 4:2191
Shalav A, Richards BS, Trupke T, Kramer KW, Gudel HU (2005) Appl Phys Lett 86:013505
Deng K, Gong T, Hu L, Wei X, Chen Y, Yin M (2011) Opt Express 19:1749
Downing E, Hesselink L, Ralston J, Macfarlane R (1996) Science 273:1185
Walsh BM, Barnes NP, Petros M, Yu JR, Singh UN (2004) J Appl Phys 95:3255
Lim SF, Riehn R, Ryu WS, Khanarian N, Tung CK, Tank D, Austin RH (2006) Nano Lett 6:169
Chen F, Bu W, Zhang S, Liu X, Liu J, Xing H et al (2011) Adv Funct Mater 21:4285
Xiao Q, Bu W, Ren Q, Zhang S, Xing H, Chen F et al (2012) Biomaterials 33:7530
Suyver JF, Aebischer A, Biner D, Gerner P, Grimm J, Heer S, Kramer KW, Reinhard C, Gudel HU (2005) Opt Mater 27:1111
Suyver JF, Grimm J, van Veen MK, Biner D, Krämer KW, Güdel HU (2006) J Lumin 117:1
Menyuk N, Dwight K, Pierce J (1972) Appl Phys Lett 21:159
Li C, Quan Z, Yang J, Yang P, Lin J (2007) Inorg Chem 46:6329
Heer S, Kömpe K, Güdel HU, Haase M (2004) Adv Mater 16:2102
Cao C, Zhang X, Chen M, Qin W, Zhang J (2010) J Alloy Compd 505:6
Wang Q, Tan MC, Zhuo R, Kumar GA, Riman RE (2010) J Nanosci Nanotechnol 10:1685
Liu J-N, Bu W, Pan L-M, Zhang S, Chen F, Zhou L et al (2012) Biomaterials 33:7282
Chen F, Bu W, Zhang S, Liu J, Fan W, Zhou L et al (2012) Adv Funct Mater. doi:10.1002/adfm.201201469
Guo J, Ma F, Gu S, Shi Y, Xie J (2012) J Alloy Compd 523:161
Liang LF, Wu H, Hu HL, Wu MM, Su Q (2004) J Alloy Compd 368:94
Zeng S, Ren G, Xu C (2011) J Alloy Compd 509:2540
Teshima K, Lee S, Shikine N, Wakabayashi T, Yubuta K, Shishido T, Oishi S (2011) Cryst Growth Des 11:995
Suzuki S, Teshima K, Wakabayashi T, Nishikiori H, Yubuta K, Shishido T, Oishi S (2011) Cryst Growth Des 11:4825
Suzuki S, Teshima K, Wakabayashi T, Nishikiori H, Ishizaki T, Oishi S (2011) J Mater Chem 21:13847
Zhang X, Yang P, Li C, Wang D, Xu J, Gai S, Lin J (2011) Chem Commun 47(44):12143
Zeng JH, Su J, Li ZH, Yan RX, Li YD (2005) Adv Mater 17:2119
Liang X, Wang X, Zhuang J, Peng Q, Li Y (2007) Adv Funct Mater 17:2757
Zhang F, Wan Y, Yu T, Zhang F, Shi Y, Xie S, Li Y, Xu L, Tu B, Zhao D (2007) Angew Chem Int Ed 46:7976
Mao Y, Park T-J, Zhang F, Zhou H, Wong SS (2007) Small 3:1122
Cao C, Yang HK, Chung JW, Moon BK, Choi BC, Jeong JH, Kim KH (2011) J Am Ceram Soc 94:3405
Boyer J-C, Cuccia LA, Capobianco JA (2007) Nano Lett 7:847
Wang F, Liu X (2008) J Am Chem Soc 130:5642
Ajayaghosh A, Varghese R, Mahesh S, Praveen VK (2006) Angew Chem Int Ed 45:7729
Yi GS, Chow GM (2006) Adv Funct Mater 16:2324
Ehlert O, Thomann R, Darbandi M, Nann T (2008) ACS Nano 2:120
Auzel F (2003) Chem Rev 104:139
Li C, Zhang C, Hou Z, Wang L, Quan Z, Lian H, Lin J (2009) J Phys Chem C 113:2332
Niu N, Yang P, He F, Zhang X, Gai S, Li C, Lin J (2012) J Mater Chem 22:10889
Tian Y, Jiao X, Zhang J, Sui N, Chen D, Hong G (2010) J Nanopart Res 12:161
Wang L, Li Y (2006) Nano Lett 6:1645
Cao C, Yang HK, Chung JW, Moon BK, Choi BC, Jeong JH, Kim KH (2011) Mater Res Bull 46:1553
Tao F, Wang Z, Yao L, Cai W, Li X (2007) J Phys Chem C 111:3241
Bovero E, van Veggel FCJM (2007) J Phys Chem C 111:4529
DeShazer LG, Dieke GH (1963) J Chem Phys 38:2190
Thomas KS, Singh S, Dieke GH (1963) J Chem Phys 38:2180
Boyer J-C, Vetrone F, Cuccia LA, Capobianco JA (2006) J Am Chem Soc 128:7444
Nyk M, Kumar R, Ohulchanskyy TY, Bergey EJ, Prasad PN (2008) Nano Lett 8:3834
Acknowledgements
This study is supported by the National Natural Science Foundation of China (Grant No. 20901040/B0111), the Key University Science Research Project of Jiangsu Province (No.10KJA430016), the Innovation Foundation for Graduate Students of Jiangsu Province China (CXLX11_0355) and a project funded by the Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, M., Lu, C., Cao, L. et al. Facile synthesis of β-NaYF4:Ln3+ (Ln = Eu, Tb, Yb/Er, Yb/Tm) microcrystals with down- and up-conversion luminescence. J Mater Sci 48, 4989–4998 (2013). https://doi.org/10.1007/s10853-013-7285-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7285-x