Abstract
Al18B4O33:Eu3+, Tb3+ whiskers have been successfully prepared by a simple gel nano-coating method using aluminum isopropoxide as the starting materials. X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), selected area electron diffraction (SAED), photoluminescence (PL), and thermogravimetric analysis (TGA) were used characterize the samples. The results show coexistence of the crystal phase Al18B4O33, amorphous phase, and Eu3+, Tb3+ ions of the samples with initial addition Al/B ratios from 3 to 1 are incorporated into the amorphous phase. The Al18B4O33:Eu3+, Tb3+ whiskers are very straight with an average diameter of 600 nm and lengths ranging from 5 to 10 μm. Under ultraviolet excitation at 365 nm, samples show mainly exhibit the characteristic emission of Eu3+ corresponding to \( ^{ 5} {\text{D}}_{ 0} \to {\text{F}}_{ 1 , 2} \) transitions due to an efficient energy transfer occurs from Tb3+ to Eu3+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rare-earth-activated inorganic phosphor materials have been widely used in modern lighting and display fields, such as fluorescent lamps, cathode-ray tubes, field emission displays for their well-defined transitions within the 4f electronic shells [1–5]. As the most frequently used activator ions in luminescent materials, the Eu3+ and Tb3+ are excellent activator which mainly show emissions due to transitions of 5D0 to 7F J (J = 1, 2, 3, 4) in the red region and 5D4 to 7F J (J = 6, 5, 4, 3) in the green region, respectively [6, 7]. Moreover, it is well-known that an effective energy transfer can take place from Tb3+ to Eu3+ in several hosts [8–10].
Borates have been proved to be useful in optical materials because of their high stability, low synthesis temperature, and their high ultraviolet (UV) transparency and nonlinear properties [11]. Aluminum borate (Al18B4O33) is a promising ceramic materials in this family for excellent mechanical properties, chemical inertness, low thermal expansion coefficient, and high-temperature stability [12–15]. In previous study, Al18B4O33 containing rare earth elements (Ce3+, Tb3+, Eu3+) have been reported [16–18]. However, so far little attention has been paid to synthesis and luminescent properties of Eu3+ and Tb3+ co-doped Al18B4O33 and the corresponding energy transfer from Tb3+ to Eu3+ in Al18B4O33 glass–ceramic composites has not been realized and reported. On the other hand, luminescence of amorphous materials was paid little attention. This is due to the fact that the quantum yield of an amorphous material is not so high as those of a well-defined crystalline family. However, glasses are well-known in optically pumped laser materials containing Nd3+ [19].
Accordingly in this article, we report the synthesis of Al18B4O33:Eu3+, Tb3+ crystalline-amorphous material by nano-coating method. Comparing with the previous reports [16–18], our present study is very different. We added boric acid to the aluminum isopropoxide alcohol solution to ensure boric acid and aluminum isopropoxide in the isopropyl alcohol react completely. Subsequently, the Al3+ in aluminum isopropoxide combines with boric acid during Lewis acid–base reaction to give amorphous aluminum borate. When the deionized water was added to the mixture dropwise, the remnants of aluminum isopropylate which undergo hydrolysis and polycondensation reactions can be utilized to coat a stable layer of alumina on amorphous aluminum borate containing of Eu3+ and Tb3+. After calcinating at higher temperatures, the glass–ceramic Al18B4O33:Eu3+, Tb3+ phase is thus obtained. The gel nano-coating method enhances mechanical properties and structural stability of the glass–ceramic Al18B4O33:Eu3+, Tb3+ and makes rare earth elements uniformly disperse in the final product. Moreover, we investigated the energy transfer property from Tb3+ to Eu3+ in Al18B4O33 crystalline-amorphous material.
Experimental
Sample preparation
The starting materials include the following chemicals: H3BO3 (99.9%), Eu2O3 (99.99%), Tb2O3 (99.99%), and aluminum isopropoxide (99.99%). Aluminum isopropoxide was synthesized from high-purity aluminum metal and isopropanol, and then purified by a followed controlled distillation process [20]. The detailed synthesis procedures were described as follows: Tb2O3, Eu2O3 (molar ratio Eu/(Al + B) = 0.01, Tb/(Al + B) = 0.01) and 0.05 mol aluminum isopropoxide were premixed in 50 mL isopropanol solvent under stirring, followed by the addition of different molar H3BO3. The deionized water was added to the vigorously stirred mixture dropwise until aluminum isopropoxide hydrolyzed completely, and then formed a gel precursor. It was dried at 60 °C for 4 h and further calcined for 3 h at 1,250 °C in an alumina crucible.
Characterization
The as-synthesized samples were examined by XRD measurement on a Rigaku-DMax 2400 diffractometer equipped with graphite monochromatized CuKα (λ = 1.5406 Å). Infrared spectroscopy (IR) was measured on a JASCO460 plus spectrophotometer. The excitation and emission spectra were recorded on a JASCO FP-6300 spectrofluorometer. The morphology of the samples was characterized by scanning electron microscopy (SEM, JSM-5600LV, JEOL). The TEM observation was carried out using a Philips TECNAI G2 s-twin microscope. The formation of the different crystalline phrases was analyzed by TGA. Thermal analyses were carried out at 10 °C min−1 under air atmosphere on Mettler Toledo apparatuses (TG/DTA 815e).
Results and discussion
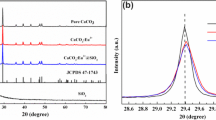
XRD patterns of the samples with initial addition Al/B molar ratio varying from 4.5:1 to 1:1 are shown in Fig. 1. The pattern of the samples with initial addition Al/B = 4.5:1 shows that there are strong diffraction peaks of Al18B4O33 phase (JCPDS 32-0003) along with weak diffraction peaks of Al2O3 (JCPDS 1-1307), EuBO3 (JCPDS 13-0485) and TbBO3 (JCPDS 24-1272). Both EuBO3 and TbBO3 exhibit very similar X-ray diffraction patterns with hexagonal-vaterite structure. This result indicates that as-synthesized samples are a mixture of Al18B4O33, Al2O3, EuBO3, and TbBO3, in which Eu3+ and Tb3+ ions have not build into the Al18B4O33 host lattice without change of its surroundings.
As the initial addition Al/B molar ratio from 3:1 to 1:1, the diffraction peaks of Al2O3, EuBO3, and TbBO3 crystalline phases disappear. Only the crystal phase of Al18B4O33 is observed. This result indicates that a great change of Eu3+ and Tb3+ surroundings occurs with an increase of H3BO3 concentration.
From the XRD results mentioned above, it seems impossible that Eu3+ and Tb3+ ions are incorporated into the crystal lattice of Al18B4O33, because Eu3+ and Tb3+ ions have a much larger radius, compared with Al3+ and B3+ ions.
Therefore, it can be inferred that the increase of H3BO3 concentration may give rise to an increase of amorphous phase, and Eu3+, Tb3+ ions dope into amorphous phase. This finding is similar the previously result reported in [16].
SEM micrograph given in Fig. 2a, b shows the morphology of Eu3+ and Tb3+ co-doped Al18B4O33 whiskers with initial addition molar ratio Al/B = 1:1. Large amounts of whiskers twist around one another to form a large aggregate (Fig. 2a). A closer examination of the samples indicates that the interweaving whiskers are very straight with an average diameter of 600 nm and lengths ranging from 5 to 10 μm.
The microscopic structure of Eu3+ and Tb3+ co-doped Al18B4O33 whiskers with initial addition molar ratio Al/B = 1:1 is further characterized by the TEM technique accompanied by SAED. TEM observations (Fig. 3a) showed that there are two types of particles A and B. Particle A has thin film structure, while particle B shows thick structure. The selected area electron diffraction patterns taken from a white square were found to have diffraction points and diffuse rings. It is strongly suggested that amorphous phase and crystalline phase coexist in the samples and Eu3+ and Tb3+ are doped into the amorphous phase. It should be noted that there are weak diffraction rings in the amorphous particle A, indicating that a small of crystallites exist.
Al18B4O33:Eu3+, Tb3+ whiskers with initial addition molar ratio Al/B = 1:1 show a red–orange emission under UV excitation. Figure 4 shows the excitation and emission spectra of the sample. The excitation spectrum of samples monitored at 613 nm is composed of two excitation bands of Eu3+ and Tb3+. The f–f transitions within Eu3+ 4f6 electron configuration (main peaks at the 365, 383, 396, 406, 468 nm) is corresponding to the electron transitions from the 7F0 ground state to 5D4, 5L7, 5L6, 5D3, 5D2), with \( ^{ 5} {\text{F}}_{ 0} \to {}^{5}{\text{L}}_{ 6} \)(396 nm) transition as most intense peak [21]. The characteristic f–f transition lines within the Tb3+ 4f8 configuration is assigned as the transitions from the 7F6 ground state to the different excited states of Tb3+, that is, 374 nm (5G6) 490 nm (5D4), respectively [22]. The emission spectrum of samples (Fig. 4b) under 365 nm excitation consists of four bands. Besides Eu3+ emissions, we can also observe the characteristic emissions of Tb3+. The peaks at about 489, 541 nm are due to Tb3+ transitions from 5D4 to 7F J (J = 6, 5), and the other emission peaks at about 591, 613 nm are from the 5D0 to 7F J (J = 1, 2). Among these luminescence emission peaks, the emission spectrum is dominated by the hypersensitive red transition at 613 nm. The most prominent emission line at 613 nm is due to hypersensitive electronic dipole transition of \( ^{ 5} {\text{D}}_{ 0} \to {}^{2}F_{7} \), induced by the lack of inversion symmetry at Eu3+ local sites, while the emission near 591 nm is the magnetic dipole transition owing to the \( ^{ 5} {\text{D}}_{ 0} \to {}^{7}F_{1} \) states, which obey the selection rule (Δj = 0, ±1), and its intensity hardly varies with evolution of the surroundings [18].
Figure 5 shows the emission spectra of Al18B4O33:Eu3+, Tb3+ with initial addition Al/B varying from 4.5 to 1 under 365 nm excitation. With the increase of H3BO3 concentration, the intensity of Eu3+ and Tb3+ emissions become stranger. Meanwhile, Eu3+ and Tb3+ emission lines become inhomogeneous and broadened. This characteristic feature of Eu3+ and Tb3+ emission spectra is quite similar to that of Eu3+ and Tb3+ in an amorphous has a characteristic features: inhomogeneous broadening of luminescence band [23]. When initial addition Al/B ratio changes from 4.5 to 1, this change of luminescent properties reveals a variation of Eu3+ and Tb3+ surroundings, indicating that Eu3+ and Tb3+ of the samples dope into an amorphous phase with the increase of H3BO3 quantity. Moreover, the emission from the 5D3 level of Tb3+ has not been observed in this sample, which can be explained by the quenching of the efficient multiphonon de-excited with lattice vibration due to the high energy of phonons in the borates [17].
The emission spectrum of Al18B4O33:Eu3+, Tb3+, Al18B4O33:Eu3+, and Al18B4O33:Tb3+ (initial addition molar ratio Al/B = 1:1) under 365 nm excitation are presented in Fig. 6 for comparison. In the Al18B4O33:Tb3+ samples, only the characteristic emissions of Tb3+ are observed. With the doping of Eu3+ and Tb3+ in the Al18B4O33, besides Tb3+ emission, we can also observe the characteristic emissions of Eu3+. With the addition Eu3+, the luminescence of Tb3+ decrease (Fig. 6), and that of Eu3+ increases, both due to the enhancing the probability of energy transfer from Tb3+ to Eu3+. All results above indicate an efficient energy transfer behavior shows that Al18B4O33:Eu3+, Tb3+ is not a mixture of TbBO3 and EuBO3, but aluminoborate glass phase, in which Eu3+ and Tb3+ has been incorporated the aluminoborate glass phase at certain H3BO3 concentration. Otherwise, the \( {\text{Tb}}^{ 3+ } \to Eu^{3 + } \)energy transfer can not occur in the separated phases [10]. A summary of emission and energy transfer process of Eu3+ and Tb3+ in Al18B4O33 is described in below. First, electrons on Tb3+ ions are excited from the ground state (4f8) to the excited state (4f75d) by UV light. Subsequently, these electrons relax to the lowest excited state 5D4 through multiphonon relaxation then either return to the ground state to produce the emissions (\( {}^{ 5}{\text{D}}_{ 4} \to {}^{7}F_{6,5,4,3} \)) or transfer their excitation energy from 5D4 (Tb3+) level to the higher excited energy levels of Eu3+ (4f6) through cross relaxation, which relax to the 5D0 (Eu3+) level, where the red–orange emission (\( {}^{ 5}{\text{D}}_{ 0} \to {}^{7}F_{0,1,2,3,4,5,6} \)) takes place [24]. Because the \( {}^{ 5}{\text{D}}_{ 4} \to {}^{7}F_{6,5,4,3} \) emission of Tb3+ is effectively overlapped with the \( {}^{ 7}{\text{F}}_{ 0 , 1} \to {}^{5}D_{0,1,2} \) absorption of Eu3+, the energy transfer from Tb3+ to Eu3+ is very efficient in general.
Figure 7 represents the TGA analysis of amorphous aluminum borate containing of Eu3+ and Tb3+. The TGA curves present two stages of weight loss and the total weight loss of about 31.5%. The initial weight loss between 30 and 210 °C results from desorption of isopropoxide alcohol and boric acid from the surface of amorphous precursors. During this process, H3BO3 will decompose into B2O3 at about 185 °C, and then B2O3 will melt at about 430 °C. There is no structural change till 750 °C. It is assigned to the glass–ceramic Al4B2O9:Eu3+, Tb3+ without any weight variation. The weight loss was detected at around 1,150–1,400 °C, which corresponds to melting of Al4B2O9 phase and a yield of Al18B4O33 and liquid [25].
Conclusion
In summary, we have demonstrated that a simple and mild gel nano-coating method for synthesis of the glass–ceramic Al18B4O33:Eu3+, Tb3+ whiskers. The results reveal coexistence of the crystal phase Al18B4O33 and aluminoborate glass phase and Eu3+, Tb3+ ions are incorporated into the aluminoborate glass phase at certain H3BO3 concentration. Al18B4O33:Eu3+, Tb3+ exhibits a red–orange emission spectrum, consisting of four emission bands peaking at 489, 541, 591, and 613 nm. With initial addition Al/B molar ratio change from 4.5 to 1, Eu3+ and Tb3+ emission intensity increase. An efficient energy transfer can occur from Tb3+ to Eu3+ in the glass–ceramic Al18B4O33:Eu3+, Tb3+ whiskers, which are ascribed to the energy overlap between Eu3+ and Tb3+. Further study is under way to modify luminescent color by controlling the doping concentration of Eu3+ and Tb3+ ions, for different composition of emissions of Eu3+ and Tb3+ resulted from energy efficiency at different doping concentration of Eu3+ and Tb3+.
References
Yu M, Lin J, Fu J, Zhang HJ (2003) J Mater Chem 13:1413
Capobianco JA, Vetrone F, Boyer JC, Speghini A, Bettinelli M (2002) Opt Mater 19:259
Evans RC, Carlos LD, Douglas P, Rocha J (2008) J Mater Chem 18:1100
Dotsenko VP, Berezovskaya IV, Efryushina NP (2010) J Mater Sci 45:1469. doi:10.1007/s10853-009-4104-5
Shan ZF, Chen DQ, Yu YL, Huang P, Lin H, Wang YS (2010) J Mater Sci 45:2775. doi:10.1007/s10853-010-4266-1
Blasse G, Grabmaier BC (1994) Luminescent materials. Springer-Verlag, Berlin
Silva ACS, Souza GG, Nobre MAL (2010) J Mater Sci 45:4216. doi:10.1007/s10853-010-4516-2
Holloway WW, Kestigian M, Newman R (1963) Phys Rev Lett 11:458
Chen W, Sammynaiken R, Huang Y (2000) J Appl Phys 88:1424
Di WH, Wang XJ, Zhu PF, Chen BJ (2007) J Solid State Chem 180:467
Wang YH, Li XX (2006) J Electrochem Soc 153:238
Suganuma K, Fujita T, Suzuki N, Niihara K (1990) J Mater Sci Lett 9:633
Ma R, Bando Y, Sato T, Tang C, Xu F (2002) J Am Chem Soc 124:10668
Ma R, Bando Y, Sato T (2002) Appl Phys Lett 81:3467
Liu Y, Li Q, Fan S (2003) Chem Phys. Lett 375:632
You HP, Hong GY (1999) J Phys Chem Solids 60:325
You HP, Hong GY, Wu XY (2003) Chem Mater 15:2000
Elssfah E, Tang CC (2007) J Phys Chem C 111:8176
Thomas LM, Stephen AP, Gary DW (1992) J Non-Cryst Solids 151:183
Ning GL, Lin Y, Lv BL (1997) J Dalian Univ Technol 37:269
Balda R, Fernádez J, Adam JL, Arriandiaga MA (1996) Phys Rev B 54:12076
Thomas KS, Singh S, Dieke GH (1963) J Chem Phys 38:2180
Tanabe S, Hirao K, Soga T, Hanada T (1992) J Solid State Chem 97:481
Nakazawa E, Shionoya S (1967) J Chem Phys 47:3211
Gielisse PJM, Foster WR (1962) Nature 69:195
Acknowledgements
The authors gratefully acknowledge support of National Natural Science Foundation of China (No. 20772014), National High Technology Research and Development Program of China (863 Program) (No. 2008AA03A325) and Young Teachers’ Training Fund of Dalian University of Technology (No. 893377).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wang, J., Ning, G., Gong, W. et al. Synthesis and luminescence properties of a novel Eu3+, Tb3+ co-doped Al18B4O33 whiskers by a gel nano-coating method. J Mater Sci 46, 1259–1263 (2011). https://doi.org/10.1007/s10853-010-4908-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4908-3