Abstract
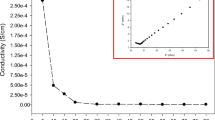
Blends of poly(vinyl chloride)-poly(methyl methacrylate) (PVC/PMMA) and poly(vinyl chloride)-poly(ethylene oxide) (PVC/PEO) with lithium triflate (LiCF3SO3) as salt, ethylene carbonate (EC), and dibuthyl phthalate (DBP) as plasticizers and nano-sized silica (SiO2) as filler, the first of its kind in such a study, were prepared using the solution-cast technique. This study affirmed that SiO2 added PVC-PMMA and PVC-PEO-blend-based polymer electrolytes have the ability to retain their ionic conductivity and integrity even after 60 days of storage time at room temperature. The reduction of ionic conductivity values in PVC-PMMA-LiCF3SO3-DBP-EC:SiO2-based and SiO2-free membranes are 9 and 30%, respectively. When PVC-PEO-blend was used, the reduction of ionic conductivity values in PVC-PEO-LiCF3SO3-DBP-EC:SiO2-based and SiO2-free system was 16 and 40%, respectively, after 60 days of storage also at room temperature. The SiO2-based complexes were also found to maintain their conductivity at higher temperatures of 60 °C and 90 °C with progressive storage times. This clearly shows that the SiO2-induced stabilizing effect is maintained even at higher temperatures. Silica has brought the conductivity of polymer electrolytes into the useful realm for materials in lithium polymer battery applications.








Similar content being viewed by others
References
Gray FM (1997) Polymer electrolytes. The Royal Society of Chemistry, Cambridge, UK
Hou X, Siow KS (2001) Polymer 42:4181
Latham RJ, Linford RG, Pynenburg R, Schlindwein WS (1992) Electrochim Acta 37:1529
Walls HJ, Zhou J, Yerian JA, Fedkiw PS, Khan SA, Stowe MK, Baker GL (2000) J Power Sources 89:156
Carriere D, Barboux P, Chaput F, Spalla O, Boilet JP (2001) Solid State Ionics 145:141
Quartarone E, Mustarelli P, Magistris A (1998) Solid State Ionics 110:1
Appetecchi GB, Passerini S (2000) Electrochim Acta 45:2139
Qiu W, Ma X, Yang Q, Fu Y, Zong X (2004) J Power Sources 138:245
Laachachi A, Cochez M, Ferriol M, Lopez-Cuesta JM, Leroy E (2005) Mater Lett 59:36
Ahmad S, Bohidar HB, Ahmad S, Agnihotry SA (2006) Polymer 47:3583
Capuano F, Croce F, Scrosati B (1991) Electrochem Soc 138:1918
Appetecchi GB, Crose F, Romagnoli P, Scrosati B, Heider U, Oesten R (1999) Electrochem Commun 1:83
Appetecchi GB, Romagnoli P, Scrosati B (2001) Electrochem Commun 3:281
Kim CS, Oh SM (2001) Electrochim Acta 46:1323
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh, S., Arof, A.K. A study incorporating nano-sized silica into PVC-blend-based polymer electrolytes for lithium batteries. J Mater Sci 44, 6404–6407 (2009). https://doi.org/10.1007/s10853-009-3883-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3883-z