Abstract
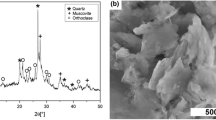
ZSM-5 aggregates were synthesized with the silica source tetraethylorthosilicate (TEOS) being hydrolyzed at acidic conditions to produce siliceous precursors, followed by the addition of aluminum sulfate and tetrapropyl ammonium bromide (TPABr), and with the resulting gel mixture being hydrothermally crystallized at basic conditions. The obtained products were characterized by XRD, SEM, and N2 adsorption. Well crystallized ZSM-5 can be successfully synthesized through the sulfuric acid-catalyzed hydrolysis of TEOS within a short crystallization time 35 h. The thus produced micro-sized single-crystal-like zeolite ZSM-5 aggregates (10 μm) are made of uniformly distributed nanocrystals with sizes of about 200 nm. Moreover, by adjusting the hydrothermal reaction parameters, such as increasing the crystallization temperature, the TPABr/SiO2 ratio and the pH value of reaction solution, the crystallization is accelerated substantially. Also, the moderate H2O/SiO2 molar ratio of 20–60 in the synthesis mixture can lead to pure ZSM-5, and yet the optimal ratio is 40.









Similar content being viewed by others
References
Argauer RJ, Landolt GR (1972) US Patent 3702866
Derouane EG, Determmerie S, Gabelica Z, Blom N (1981) Appl Catal 1:201
Hölderich W, Hesse M, Näumann F (1988) Angew Chem Int Ed 27:226
Kumar N, Lindfors LE, Byggningsbacka R (1996) Appl Catal A Gen 139:189
Byggningsbacka R, Kumar N, Lindfors LE (1998) J Catal 178:611
Rajagopalan K, Peters AW, Edwards GC (1986) Appl Catal 23:69
Camblor MA, Corma A, Martínez A, Mocholí FA, Pariente JP (1989) Appl Catal 55:65
Vogel B, Schneider C, Klemm E (2002) Catal Lett 79:107
Davis ME (2002) Nature 417:813
Zheng Z, Hall AS, Guliants VV (2008) J Mater Sci 43:2499. doi:https://doi.org/10.1007/s10853-008-2560-y
Tosheva L, Valtchev VP (2005) Chem Mater 17:2494
Mintova S, Voltchev V (2002) Microporous Mesoporous Mater 55:171
Mohamed RM, Aly HM, El-Shahat MF, Ibrahim IA (2005) Microporous Mesoporous Mater 79:7
Reding G, Mäurer T, Kraushaar-Czarnetzki B (2003) Microporous Mesoporous Mater 57:83
Li ZJ, Li S, Luo HM, Yan YS (2004) Adv Funct Mater 14:1019
Cooper CA, Lin YS (2007) J Mater Sci 42:320. doi:https://doi.org/10.1007/s10853-006-1020-9
Grieken RV, Sotelo JL, Menéndez JM, Melero JA (2000) Microporous Mesoporous Mater 39:135
Aguado J, Serrano DP, Escola JM, Rodríguez JM (2004) Microporous Mesoporous Mater 75:41
Li Q, Mihailova B, Creaser D, Sterte J (2000) Microporous Mesoporous Mater 40:53
Li Q, Mihailova B, Creaser D, Sterte J (2001) Microporous Mesoporous Mater 43:51
Tuel A, Taârit YB (1994) Zeolites 14:594
Tan B, Rankin SE (2006) J Phys Chem B 110:22353
Wu YJ, Ren XQ, Wang J (2009) Mater Chem Phys 113:773
Wu YJ, Ren XQ, Lu YD, Wang J (2008) Mater Lett 62:317
Brinker CJ, Scherer GW (1985) J Non-Cryst Solids 70:301
Sanchez J, McCormick A (1992) J Phys Chem 96:8973
Šefčík J, McCormick AV (1997) Catal Today 35:205
Serrano DP, Uguina MA, Sanz R, Castillo E, Rodríguez A, Sánchez P (2004) Microporous Mesoporous Mater 69:197
Ding L, Zheng Y, Zhang Z, Ring Z, Chen J (2006) Microporous Mesoporous Mater 94:1
Madhusoodana CD, Das RN, Kameshima Y, Okada K (2005) J Porous Mat 12:273
Thompson RW (1998) Mol Sieves 1:1
Phiriyawirut P, Magaraphan R, Jamieson AM, Wongkasemjit S (2003) Mater Sci Eng A 361:147
Fouad OA, Mohamed RM, Hassan MS, Ibrahim IA (2006) Catal Today 116:82
Mohamed MM, Zidan FI, Fodail MH (2007) J Mater Sci 42:4066. doi:https://doi.org/10.1007/s10853-006-0172-y
Lok BM, Cannan TR, Messina CA (1983) Zeolites 3:282
McCormick AV, Bell AT (1989) Catal Rev Sci Eng 31(1–2):97
Persson AE, Schoeman BJ, Sterte J, Otterstedt JE (1995) Zeolites 15:611
Gabelica Z, Derouane EG, Blom N (1983) Appl Catal 5:109
Suzuki K, Kiyozumi Y, Matsuzaki K, Shin S (1987) Appl Catal 35:401
Ko YS, Ahn WS (2004) Powder Technol 145:10
Thoma SG, Nenoff TM (2000) Microporous Mesoporous Mater 41:295
Kim SD, Noh SH, Seong KH, Kim WJ (2004) Microporous Mesoporous Mater 72:185
Acknowledgements
This study is supported by the Key Natural Science Foundation for Universities of Jiangsu Province (06KJA53012), National Natural Science Foundation of China (No. 20776069) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT 0732).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gu, J., Wu, Y., Wang, J. et al. In situ assembly of ZSM-5 nanocrystals into micro-sized single-crystal-like aggregates via acid-catalyzed hydrolysis of tetraethylorthosilicate. J Mater Sci 44, 3777–3783 (2009). https://doi.org/10.1007/s10853-009-3508-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3508-6