Abstract
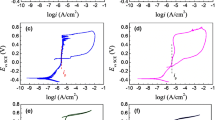
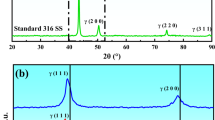
Corrosion behavior and chemical structure of the passive film of a newly developed 200 series austenitic stainless steel (216L) were studied in sulfuric acid (H2SO4) and compared with 316L. From potentiodynamic polarization studies it was found that the corrosion behavior of 216L closely follows that of 316L. The breakdown of passivity was evaluated by addition of sodium chloride (NaCl). The immersion tests revealed that the corrosion rate of 216L in various concentrations of H2SO4 at ambient temperature is equivalent to 316L. X-ray photoelectron spectroscopy (XPS) analysis of the passive film formed on 216L revealed enrichment of Cr ions on the surface while Mo and N compounds were also present. Ni and Mn ions were conspicuous by their absence in the passive film.










Similar content being viewed by others
References
Metal Bulletin, World Steel & Metal News. No. 8978, 15 January 2007, p 15
International Stainless Steel Forum (February 2008) E&S Bulletin. No 10 Shanghi, p 2
Singhal LK (2005) Iron Steel Rev 4:68
Fourie JW, Bentley AP (1987) In: Proceedings of the conference on manganese containing stainless steels, Cincinnati, OH, October 1987, pp 10–15
Ahila S, Reynders B, Grabke HJ (1996) Corros Sci 38:1991. doi:https://doi.org/10.1016/S0010-938X(96)00092-3
Toor I-u-H, Park HJ, Kwon HS (2008) Corros Sci 50:404. doi:https://doi.org/10.1016/j.corsci.2007.07.004
Sedriks AJ (1996) Corrosion of stainless steels. Wiley Inter science, New York
Chivinski JA (February 1972) Metal progress, 55
Kearns JR (1985) In: Lula RA (ed) New development in stainless steel technology. ASM, Ohio, pp 117–128
Qiu JH (2002) Surf Interface Anal 33:830. doi:https://doi.org/10.1002/sia.1460
Wagner CD, Riggs WM, Davis LE, Moulder JF, Muilenberg GE (1979) Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corporation, Physical Electronics, Minnesota, p 72
Grimal JM, Marcus P (1992) Corros Sci 33:805. doi:https://doi.org/10.1016/0010-938X(92)90113-H
Stypula B, Stoch J (1994) Corros Sci 36:2159. doi:https://doi.org/10.1016/0010-938X(94)90014-0
Beccaria AM, Castello G, Poggi G (1995) Br Corros J 30:283
Latha G, Rajendran N, Rajeswari S (1997) J Mater Eng Perform 6:743. doi:https://doi.org/10.1007/s11665-997-0076-2
Loechel BP, Strehblow HH (1984) J Electrochem Soc 131:713. doi:https://doi.org/10.1149/1.2115678
Tetsuya T, Yoshimasa I, Yoshimtsu O (1997) Mater Trans JIM 38:78
Nefedov VI, Salyn YV, Leonhardt G, Scheibe R (1977) J Electron Spectrosc Relat Phenom 10:121. doi:https://doi.org/10.1016/0368-2048(77)85010-X
Sadough Vanini A, Audouard JP, Marcus P (1994) Corros Sci 36:1825. doi:https://doi.org/10.1016/0010-938X(94)90021-3
Kovac CA, Clabes JG, Goldberg MJ (1988) Vac Sci Technol A 6:991. doi:https://doi.org/10.1116/1.575006
Borgmann D, Hums E, Hopfengartner G, Wedler G, Spitznagel GW, Rademacher I (1993) J Electron Spectrosc Relat Phenom 63:91. doi:https://doi.org/10.1016/0368-2048(93)80042-K
Olefjord I, Brox B, Jelvestam U (1985) J Electrochem Sci Tech (Paris) 132:2854
Leygraf C, Hultquist G, Olefjord I, Elfstrom BO (1978) Corros Sci 19:343. doi:https://doi.org/10.1016/0010-938X(79)90026-X
Werfel F, Brummer O (1983) Phys Scr 28:92. doi:https://doi.org/10.1088/0031-8949/28/1/013
Dickinson T, Povey AF, Sherwood PMA (1976) J Chem Soc Faraday Trans I 72:686. doi:https://doi.org/10.1039/f19767200686
Pashutski A, Folman M (1989) Surf Sci 216:395. doi:https://doi.org/10.1016/0039-6028(89)90383-X
Mustin C, De Donato PH, Benoit R, Erre R (1993) Appl Surf Sci 68:147
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shankar Rao, V., Singhal, L.K. Corrosion behavior and passive film chemistry of 216L stainless steel in sulphuric acid. J Mater Sci 44, 2327–2333 (2009). https://doi.org/10.1007/s10853-008-2976-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2976-4