Abstract
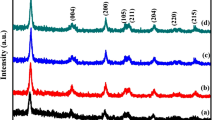
Sm3+-doped TiO2 nanocrystalline was synthesized by a sol–gel auto-combustion method and characterized by X-ray diffraction, Brunauer-Emmett-Teller method (BET), UV–vis diffuse reflectance spectroscopy (DRS), and also photoluminescence (PL) emission spectroscopy. The photocatalytic activity of Sm3+–TiO2 catalyst was evaluated by measuring degradation rates of methylene blue (MB) under either UV or visible light. The results showed that doping with the samarium ions significantly enhanced the photocatalytic activity for MB degradation under UV or visible light irradiation. This was ascribed to the fact that a small amount of samarium dopant simultaneously increased MB adsorption capacity and separation efficiency of electron-hole pairs. The results of DRS showed that Sm3+-doped TiO2 had significant absorption between 400 nm and 500 nm, which increased with the increase of samarium ion content. The adsorption experimental demonstrated that Sm3+–TiO2 had a higher MB adsorption capacity than undoped TiO2 and adsorption capacity of MB increased with the increase of samarium ion content. It is found that the stronger the PL intensity, the higher the photocatalytic activity. This could be explained by the points that PL spectra mainly resulted from surface oxygen vacancies and defects during the process of PL, while surface oxygen vacancies and defects could be favorable in capturing the photoinduced electrons during the process of photocatalytic reactions, so that the recombination of photoinduced electrons and holes could be effectively inhibited.






Similar content being viewed by others
References
Hoffmann MR, Choi ST, Martin W, Bahnemann DW (1995) Chem Rev 95:69
Fujishima A, Rao TN, Truk DA (2000) J Photochem Photobiol C: Photochem Rev 1:1
Choi W, Termin A, Hoffmann MR (1994) J Phys Chem 98:13669
Hattori A, Tokihisa Y et al (2000) J Electrochem Soc 147:2279
Wang C, Xu B-Q (2005) J Solid State Chem 178:3500
Iliev V, Tomova D et al (2006) Appl Catal B: Environ 63:266
Kim DH, Woo SI et al (2005) Solid State Commun 136:554
Li FB, Li XZ, Hou MF, Cheah KW, Choy WCH (2005) Appl Catal A: Gen 285:181
Zhang Y, Xu H, Xu Y, Zhang H, Wang Y (2005) J Photochem Photobiol A: Chem 170:279
Yan X, He J, Evans DG, Duan X, Zhu Y (2005) Appl Catal B: Environ 55:243
Xie Y, Yuan C, Li X (2005) Mater Sci Eng B 117:325
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Science 293:269
Kamisaka H, Adachi T, Yamashita K (2005) J Chem Phys 123:084704
Madhusudan Reddy K, Baruwati B, Jayalakshmi M, Mohan Rao M, Manorama SV (2005) J Solid Chem 178:3352
Li W, Wang Y, Lin H, Ismat Shah S, Doren CP, Rykov SA, Chen JG, Barteau MA (2003) Appl Phys Lett 83:4143
Xie YB, Yuan CW (2004) Appl Surf Sci 221:17
Spurr RA, Myers H (1957) Anal Chem 29:760
Yamashita H, Ichihashi Y, Zhang SG, Matrumura Y, Souma Y, Tatsumi T, Anpo M (1997) Appl Surf Sci 121/122:305
Zhang LD, Mo CM (1995) Nanostruct Mater 6:831
Danzhen L, Yi Z, Xianzhi F (2000) Chin J Mater Res 14:639
Jing L, Xin B, Yuan F, Xue L, Wang B, Fu H (2006) J Phys Chem B 110:17860
Hurum DC, Agrios AG, Gray KA, Rajh T, Thurnauer MC (2003) J Phys Chem B 107:4545
Miyagi T, Kamei M, Mitsuhashi T, Ishigaki T, Yamazaki A (2004) Chem Phys Lett 390:399
Fujishima A, Rao TN, Tryk DA (2000) J Photochem Photobiol C: Photochem Rev 1:1
Kamat PV (1993) Chem Rev 93:267
Linsebigler AL, Lu G, Yates JT (1995) Chem Rev 95:735
Liu H, Cheng S, Wu M, Wu H, Zhang J, Li W, Cao C (2000) J Phys Chem A 104:7016
Acknowledgments
This work was supported by the Provincial Excellent PhD Thesis Research Program of Hunan (No.2004-141) and the Postgraduate Educational Innovation Engineering of Central South University (No.2006-48). The authors are grateful to Dr. Huang Suping for her encouragement and helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, Q., Si, Z., Zhang, J. et al. Effects of samarium dopant on photocatalytic activity of TiO2 nanocrystallite for methylene blue degradation. J Mater Sci 42, 9194–9199 (2007). https://doi.org/10.1007/s10853-007-1919-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1919-9