Abstract
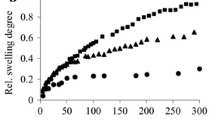
The temperature sensitive nature of poly(N-isopropylacrylamide) makes it an attractive candidate for controlled drug delivery devices. A series of temperature responsive poly (N-isopropylacrylamide)-polyvinyl pyrrolidinone random copolymers were produced by free radical polymerisation using 1-hydroxycyclohexylphenyketone as a UV-light sensitive initiator. The chemical structure of the xerogels was characterised by means of Fourier transform infrared spectroscopy (FTIR). The copolymers possess a lower critical solution temperature (LCST) in pure water, but the transition temperature may be affected by the addition of various cosolutes. The LCST of the pseudogels (physically crosslinked gels) was investigated in distilled water and a variety of salt and pH buffer solutions, using modulated differential scanning calorimetry (MDSC) and rheological analysis. The pH buffer solutions prepared mimic the variety of conditions encountered by drug delivery systems administered orally. The pH effects on the LCSTs of the temperature sensitive gels appear not obvious; while the salts used to prepare the pH buffer solutions have a more notable effect (‘salting out effect’) on the phase transition temperature. All swelling studies were carried out on the hydrogels at 37°C in distilled water, pH buffer 1.2 and pH buffer 6.8. The swelling/dissociation behaviour of the gels is found to be highly dependent on the pH buffer solution used, as the salts incorporated in preparing the pH buffer solutions lowers the phase transition of the copolymers to below the test temperature of 37°C, thus making them less soluble.









Similar content being viewed by others
References
Wise DL (2001) Handbook of Pharmaceutical Controlled Release Technology. Marcel Dekker Inc., New York-Basel
Alderborn G, Aulton M (2002) Pharmaceutics, the science of dosage form design Ed., Elsevier Science, pp 397–441
Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM (1999) Chem Rev 99:3181–3198
Park K, Qui Y (2001) Adv Drug Deliv Rev 53:321–339
Nguyen KT, West JL (2002) Biomaterials 23:4307–4314
Kishida A, Ikada Y (2002) Hydrogels for biomedical and pharmaceutical applications. In: Dumitriu S (ed) Polymeric biomaterials, 2nd ed., pp 133–45
Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Eur J Pharm Biopharm 50:27–46
Ravichandran P, Shantha KL, Panduranga Rao K (1997) Inter J Pharm 154:89–94
Kopeček J (2003) Eur J Pharmaceutical Sci 20:1–16
Bromberg LE, Ron ES (1998) Adv Drug Deliv Rev 31:197–221
LaPorte RJ (1997) Hydrophilic polymer coatings for medical devices. Technomic Pub. Co. Inc
Chilkoti A, Dreher M, Meyer D, Raucher D (2002) Adv Drug Deliv Rev 54:613–630
Grass M, Colombo I, Lapasin R (2000) J Controlled Release 68:97–113
Murata Y, Sasaki N, Miyamoto E, Kawashima S (2000) Eur J Pharmaceutics Biopharm 50:221–226
Bokias G, Staikos G, Iliopoulos I (2000) Polymer 41:7399–7405
Costa R (2002) Polymer 43:5879–5885
Liu XM, Wang LI, Wang L, Haung J, He C (2004) Biomaterials 25:5659–5666
Schild HG (1992) Prog Polym Sci 17:163–249
Deshmukh MV, Vaidya AA, Kulkarni MG, Rajamohonan PR, Ganapathy S (2000) Polymer 41:7951–7960
Otake K, Inomata H, Konno M, Saito S (1990) Macromolecules 23:283–289
Geever LM, Devine DM, Nugent MJD, Kennedy JE, Lyons JG, Hanley A, Higginbotham CL (2006) Eur Polymer J 42:2540
Geever LM, Devine DM, Nugent MJD, Kennedy JE, Lyons JG, Higginbotham CL (2006) Eur Polymer J 42:69–80
Ju HK, Kim SY, Kim SJ, Lee YM (2002) J Appl Polymer Sci 83:1128–1139
Ebril C, Kazancioğlu E, Uyanik N (2004) Eur Polymer J 40:1145–1154
Heskins M, Guillet JE (1969) J Macromol 2:1441
Schild HG, Muthukumar M, Tirrell DA (1991) Macromolecules 24:948–952
Kubota K, Fujishige S, Ando I (1990) J Phys Chem 94:5154–5158
Boutris C, Chatzi E (1997) Polymer 38:2567–2570
Feil H, Bae YH, Feijen J, Kim SW (1993) Macromolecules 26:2496–2500
Tam KC, Wu XY, Pelton RH (1992) Polymer 33:436–438
Yang H, Cheng R, Wang Z (2003) Polymer 44:7175–7180
Graziano G (2000) Int J Biol Macromol 27:89–97
Han CK, Bae YH (1998) Polymer 39:2809–2814
Ruel-Gariépy E, Leroux JC (2004) Eur J Pharm Biopharm 58:409–426
Benrebouh A, Avoce D, Zhu XX (2001) Polymer 42:4031–4038
Gan LH, Roshan Deen G, Loh XJ, Gan YY (2001) Polymer 42:65–69
Eeckman F, Amighi K, Moës AJ (2001) Int J Pharm 222:259–270
Eeckman F, Moës AJ, Amighi K (2002) Int J Pharm 241:113–125
Jones MS (1999) Eur Polymer J 35:795–801
Narasimhan B, Peppas NA (1996) Macromolecules 29:3283–3291
Devine DM, Higginbotham CL (2003) Polymer 44:7851–7860
Zhang XZ, Zhuo RX (2000) Eur Polymer J 36:643–645
Devine DM, Geever LM, Higginbotham CL (2005) J Mater Sci 40:3429–3436
Acknowledgements
This study was supported in parts by grants from both Enterprise Ireland and the Athlone Institute of Technology research and development fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geever, L.M., Nugent, M.J.D. & Higginbotham, C.L. The effect of salts and pH buffered solutions on the phase transition temperature and swelling of thermoresponsive pseudogels based on N-isopropylacrylamide. J Mater Sci 42, 9845–9854 (2007). https://doi.org/10.1007/s10853-007-1814-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1814-4