Abstract
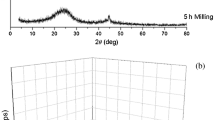
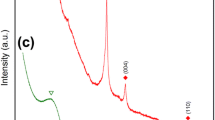
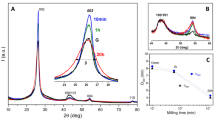
The change in graphitic carbon structure induced by mechanical milling has been monitored by Raman spectroscopy, transmission electron microscopy (TEM) and X-ray diffraction. It is well known that progressive rod milling of graphite results in an increase in structural disorder. Here, it has been found that a milling time of around 80 h is crucial in producing maximum nanocrystallite formation and this affects the nature of the products formed before or after annealing. At about 80 h equilibrium forms and no further production of nanocrystallites is possible although if additional energy is added amorphous carbon begins to form. Annealing produces different nanographitic carbons depending on the milling conditions because the material may be milled to an equilibrium concentration of nanocrystallites or less, or with additional energy transformed further past equilibrium to new product. Linear morphological structures and trace amounts of carbon nanotubes were found on milling for 80 h and annealing, but concentric layers of carbons were observed in samples milled as long as 240 h.
Similar content being viewed by others
References
K. Niwase, T. Tanaka, Y. Kakimoto, K. N. Ishihara and P. H. Shingu, Mater. Trans J.I.M. 36 (1995) 282.
T. D. Shen, WQ. Ge, K. Y. Wang, M. X. Quan, J. T. Wang and W. D. Wei, Nanostructed Materials 7 (1996).
J. Tang, W. Zhao, L. Li, A. U. Falster, W. B Simmons and W. L. Zhou, J. Mater Res 11 (1996) 733.
W. L. Zhou, Y. Ikuhara, W. Zhao and J. Tang, Carbon 33 (1995) 1177.
J. B. Aladekoma and R. H. Bragg, Carbon 28 (1990) 897.
M. Nakamizo, H. Honda and M. Inagaki, Carbon 16 (1978) 281.
T. S. Ong and H. Yang, Carbon 38 (2000) 2077.
H. Hermann, T. Schubert, W. Gruner and N. Mattern, Nanostruct. Mater. 8 (1997) 215.
T. Fukunga, K. Nagano, U. Mizutani, H. Wakayama and Y. Fukushima, J. Non-Cryst. Solids 232 (1998) 416.
N. J. Welham and J. S. Williams, Carbon 36 (1998) 1309.
J. Y. Huang, H. Yasuda and H. Mori, Chem. Phys. Lett. 303 (1999) 130.
Z. H. Chen, H. S. Yang, G. T. Wu, M. Wang, F. M. Deng, X. B. Zhang, J. C. Peng and W. Z. Li, J. Crystal Growth 218 (2000) 57.
Y. Chen, L. T. Chadderton, J. S. Williams and G. J. Fitz, Mater. Sci. Forum 343–346 (2000) 63.
Y. Chen, M. J. Conway and G. J. Fitz, Appl. Phys. A (2002) Online publication: DOI: 10.1007/s00339-002-(1986) 3.
Y. Chen, G. J. Fitz, L. T. Chadderton and L. Chaffron, J. Metastable and Nanocrystalline Mater 2–6 (1999) 375.
Y. Chen, G. J. Fitz, L. T. Chadderton and L. Chaffron, Appl. Phys. Lett. 74 (1999) 2782.
L. T. Chadderton and Y. Chen, Phys Lett A 263 (1999) 401.
S. Iijima, Nature 354 (1991) 56.
C. P. Marshall and M. A. Wilson, Carbon 42 (2004) 2179.
H. Connan, B. Reedy, C. P. Marshall and M. A. Wilson, Energy and Fuels 18 (2004) 1607.
J. Kalman, C. Nordlund, H. K. Patney, L. A. Evans and M. A. Wilson, Carbon 39 (2001) 137.
R. Birringer and H. Gleiter, in “Encylopedia of Materials Science,” edited by R. W. Cahn (Pergamon Press, 1988) vol. 1 (Supp.), p. 379.
P. J. F. Harris, Carbon 39 (2001) 909.
F. Tuingstra and J. L. Koenig, J. Chem. Phys. 53 (1970) 1126.
P. Lespade, R. Al-Jishi and M. S. Dresselhaus, Carbon 5 (1982) 427.
A. Cuesta, P. Dhamelincourt, J. Laureyns, A. Martinez-Alonso and J. M. D. Tascon, Carbon 32 (1994) 1523.
J. Dong, W. C. Shen, B. F. Zhang, F. Y. Kang, X. Liu, J. L. Gu, D. S. Li, X. F. Hu and N. P. Chen, J. Phys. Chem. Solids 62 (2001) 2047.
B. Bokhonov and M. Korchagin, J. Alloys and Compounds 333 (2002) 308.
M. Endo, C. Kim, T. Karaki, T. Kasa, M. J. Matthews, S. D. M. Brown, M. S. Dresselhaus, T. Tamaki and T. Nishimura, Carbon 6 (1998) 1633.
M. A. Wilson, G. S. K. Kannangara, G. Smith, M. Simmons and B. Raguse (Nanotechnology Chapman Hall, Boca Raton USA, 2002) p. 75.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smeulders, D.E., Milev, A.S., Kamali Kannangara, G.S. et al. Rod milling and thermal annealing of graphite: Passing the equilibrium barrier. J Mater Sci 40, 655–662 (2005). https://doi.org/10.1007/s10853-005-6303-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10853-005-6303-z