Abstract
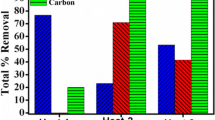
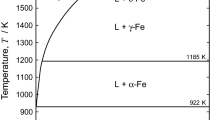
This article studies the mechanism of iron reduction in magnesium alloy melt by investigating the iron concentrations in different parts in the melt with different holding time. The iron concentrations at the top, the center and the bottom of the melt are measured with the holding time of 300, 1800 and 7200 s. The elements in the sludge settling down in the crucible are also determined by ICP. With the increasing holding time, the iron content in the upper part of the melt decreases and that in the lower part of the melt increases. And the iron content in the sludge increases rapidly. The iron concentrations in the melt and the sludge change little with longer holding time than 1800 s. Among the three iron reduction agents B2O3, MnCl2 and TiO2, B2O3 has the highest iron reduction efficiency (IRE). IRE of B2O3 is as two times and five times as IRE of MnCl2 and IRE of TiO2 respectively. Formation of FeB and settling of it into melting sludge are believed to be the primary mechanism of iron reduction by B2O3 processing. The mechanism of iron reduction in magnesium melt by MnCl2 or TiO2 processing is studied by thermodynamic analysis. Formation of substance containing MnFeAl or TiFe with high density and high melting point and settling of them into melting sludge are suggested to be the mechanism of iron reduction by MnCl2 or TiO2 processing.
Similar content being viewed by others
References
J. E. GRAY and B. LUAN, J. All. Comp. 336 (2002) 88.
H. FURUYA, N. KOGISO, S. MATUNAGA and K. SENDA, Mater. Sci. Forum 350 (2000) 341.
R. BROWN, Light Metal Age 58(9/10) (2000) 54.
R. P. PAWLEK and R. B. BROWN, ibid. 59(8) (2001) 50.
S. KLEINER, O. BEFFORT, A. WAHLEN and P. J. UGGOWITZER, J. Light Metals 2 (2002) 277.
E. B. ROBERT, Light Metal Age 61(1/2) (2003) 53.
T. HAITANI, Y. TAMURA, T. MOTEGI, N. KONO and H. TAMEHIRO, Mater. Sci. Forum 419/422 (2003) 697.
J. D. HANAWALT, Trans. AIME 147 (1942) 279.
M. INOUE, M. IWAI, K. MATUZAWA, S. KAMADO and Y. KOJIMA, J. Japan Inst. Light Metals 48(6) (1998) 257.
Y. TAMURA, T. MOTEGI, N. KONO and E. SATO, Mater. Sci. Forum 350/351 (2000) 199.
T. HAITANI, Y. TAMURA, E. YANO, T. MOTEGI, N. KONO and E. SATO, J. Japan Inst. Light Met. 51 (2001) 403.
H. GAO, G. WU, W. DING, L. LIU, X. ZENG and Y. ZHU, Mater. Sci. Engng A368 (2004) 311.
G. WU, C. ZHAI, X. ZENG, W. DING and Y. ZHU, Acta Metall. Sinica 39 (2003) 729.
G. WU, M. XIE, C. DI, X. ZENG, Y. ZHU and W. DING, Trans. Nonferr. Met. Soc. China 13 (2003) 1260.
Y. LINAG, Y. CHE and X. LIU, “Handbook of Inorganic Thermodynamic Data” (North-East University Press, Shenyang, 1993).
R. KUZMAN, “Handbook of Thermodynamic Tables and Charts” (Hemisphere Publishing Corporation, Washington, 1976).
E. T. TURKDOGAN, “Physical Chemistry of High Temperature Technology” (Academic Press, New York, 1980) p. 25.
R. XU, “Magnesium Metallurgy” (Metallurgy Industry Press, Beijing, 1993), p. 314.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, G.H., Gao, H.T., Ding, W.J. et al. Study on mechanism of iron reduction in magnesium alloy melt. J Mater Sci 40, 6175–6180 (2005). https://doi.org/10.1007/s10853-005-3161-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-005-3161-7