Abstract
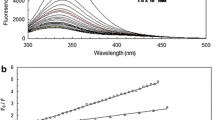
The interaction of the newly approved pharmacon esketamine with HSA (human serum albumin) has been investigated by various spectrographic methods. The fluorescence quenching results of HSA by esketamine revealed that the 1:1 ground state complex has formed. The results of binding parameters lead to the conclusion that the reaction was exothermic. In the presence of esketamine, HSA was found to undergo partial unfolding, and thermodynamic parameters (ΔG° = − 2.08 × 104 J·mol−1, ΔS° = 35.7 J·mol−1·K−1, and ΔH° = − 1.20 × 104 J·mol−1 at 298 K) revealed that electrostatic forces dominated the stabilization of the complex. The qualitative and quantitative analyses of conformational changes of HSA were conducted using circular dichroism, three-dimensional, and synchronous fluorescence spectroscopy, revealing the loosening of skeleton structure and adaptive modifications of secondary structures. Fe3+ and Mg2+ may help prolong the storage time and improve the drug efficacy.









Similar content being viewed by others
References
Daly, E.J., Singh, J.B., Fedgchin, M., Cooper, K., Lim, P., Shelton, R.C., Thase, M.E., Winokur, A., Van Nueten, L., Manji, H.: Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 75(2), 139–148 (2018)
Kim, J., Farchione, T., Potter, A., Chen, Q., Temple, R.: Esketamine for treatment-resistant depression-first FDA-approved antidepressant in a new class. N. Engl. J. Med 381(1), 1–4 (2019)
Swainson, J., Thomas, R.K., Archer, S., Chrenek, C., MacKay, M.-A., Baker, G., Dursun, S., Klassen, L.J., Chokka, P., Demas, M.L.: Esketamine for treatment resistant depression. Expert Rev. Neurother. 19(10), 899–911 (2019)
Turner, E.H.: Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry 6(12), 977–979 (2019)
Jonkman, K., Van Der Schrier, R., Van Velzen, M., Aarts, L., Olofsen, E., Sarton, E., Niesters, M., Dahan, A.: Differential role of nitric oxide in the psychedelic symptoms induced by racemic ketamine and esketamine in human volunteers. Br. J. Anaesth. 120(5), 1009–1018 (2018)
Nabati, M., Bodaghi-Namileh, V., Sarshar, S.: Molecular Modeling of the antagonist compound esketamine and its molecular docking study with non-competitive N-methyl-D-aspartate (NMDA) receptors NR1, NR2A, NR2B and NR2D. Progress Chem. Biochem. Res. 2(3), 108–119 (2019)
Yang, C., Han, M., Zhang, J.-C., Ren, Q., Hashimoto, K.: Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatry Res. 239, 281–283 (2016)
Yun, Z., Li, L., Liu, L., He, B., Zhao, X., Jiang, G.: Characterization of mercury-containing protein in human plasma. Metallomics 5(7), 821–827 (2013)
Sindhu, R., Tiwari, A.K., Mishra, L.C., Husain, M.M.: Spectroscopic interaction of a coumarin derivative with bovine serum albumin. Cancer Biother. Radiopharm. 27(7), 452–456 (2012)
Song, S., Li, Y., Liu, Q.S., Wang, H., Li, P., Shi, J., Hu, L., Zhang, H., Liu, Y., Li, K.: Interaction of mercury ion (Hg2+) with blood and cytotoxicity attenuation by serum albumin binding. Hazard Mater. 239, 281–283 (2021)
Leboffe, L., di Masi, A., Polticelli, F., Trezza, V., Ascenzi, P.: Structural basis of drug recognition by human serum albumin. Curr. Med. Chem. 27(30), 4907–4931 (2019)
Zhao, X., Lu, D., Hao, F., Liu, R.: Exploring the diameter and surface dependent conformational changes in carbon nanotube-protein corona and the related cytotoxicity. J. Hazard. Mater. 292, 98–107 (2015)
Estrada, E., Uriarte, E., Molina, E., Simon-Manso, Y., Milne, G.W.A.: An integrated in silico analysis of drug-binding to human serum albumin. J. Chem Inf. Model. 46(6), 2709–2724 (2006)
Sharif-Barfeh, Z., Beigoli, S., Marouzi, S., Rad, A.S., Asoodeh, A., Chamani, J.: Multi-spectroscopic and HPLC studies of the interaction between estradiol and cyclophosphamide with human serum albumin: binary and ternary systems. J. Solution Chem. 46(2), 488–504 (2017)
Zhao, X., Lu, D., Liu, Q.S., Li, Y., Feng, R., Hao, F., Qu, G., Zhou, Q., Jiang, G.: Hematological effects of gold nanorods on erythrocytes: hemolysis and hemoglobin conformational and functional changes. Adv. Sci. 4(12), 1700296 (2017)
Kamshad, M., Jahanshah Talab, M., Beigoli, S., Sharifirad, A., Chamani, J.: Use of spectroscopic and zeta potential techniques to study the interaction between lysozyme and curcumin in the presence of silver nanoparticles at different sizes. J. Biomol. Struct. Dyn. 37(8), 2030–2040 (2019)
Khan, A.Y., Hossain, M., Kumar, G.S.: Investigations on the interaction of the phototoxic alkaloid coralyne with serum albumins. Chemosphere 87(7), 775–781 (2012)
Rahnama, E., Mahmoodian-Moghaddam, M., Khorsand-Ahmadi, S., Saberi, M.R., Chamani, J.: Binding site identification of metformin to human serum albumin and glycated human serum albumin by spectroscopic and molecular modeling techniques: a comparison study. J. Biomol. Struct. Dyn. 33(3), 513–533 (2015)
Kitamura, M., Murakami, K., Yamada, K., Kawai, K., Kunishima, M.: Binding of sulforhodamine B to human serum albumin: a spectroscopic study. Dyes And Pigments 99(3), 588–593 (2013)
Zhao, X., Liu, R., Teng, Y., Liu, X.: The interaction between Ag(+) and bovine serum albumin: a spectroscopic investigation. Sci. Total Environ. 409(5), 892–897 (2011)
Chanphai, P., Tajmir-Riahi, H.: Tea polyphenols bind serum albumins: a potential application for polyphenol delivery. Food Hydrocoll. 89, 461–467 (2019)
Sheng, F., Wang, Y., Zhao, X., Tian, N., Hu, H., Li, P.: Separation and identification of anthocyanin extracted from mulberry fruit and the pigment binding properties toward human serum albumin. J. Agric. Food Chem. 62(28), 6813–6819 (2014)
Zhao, X., Sheng, F., Zheng, J., Liu, R.: Composition and stability of anthocyanins from purple solanum tuberosum and their protective influence on Cr(VI) targeted to bovine serum albumin. J. Agric. Food Chem. 59(14), 7902–7909 (2011)
Ross, P.D., Subramanian, S.: Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20(11), 3096–3102 (1981)
Chanphai, P., Ouellette, V., Bérubé, G., Tajmir-Riahi, H.: Conjugation of testo and testo-Pt (II) with serum proteins: loading efficacy and protein conformation. Inter. J. Boil. Macromol. 118, 1112–1119 (2018)
Beigoli, S., Sharifi Rad, A., Askari, A., Assaran Darban, R., Chamani, J.: Isothermal titration calorimetry and stopped flow circular dichroism investigations of the interaction between lomefloxacin and human serum albumin in the presence of amino acids. J. Biomol. Struct. Dyn. 37(9), 2265–2282 (2019)
Zhao, Z., Li, G., Liu, Q.S., Liu, W., Qu, G., Hu, L., Long, Y., Cai, Z., Zhao, X., Jiang, G.: Identification and interaction mechanism of protein corona on silver nanoparticles with different sizes and the cellular responses. J. Hazard. Mater. 414, 125582 (2021)
Xu, C., Zhao, X., Wang, L., Zhang, X., Wang, Y., Lan, J.: Protein conjugation with gold nanoparticles: spectroscopic and thermodynamic analysis on the conformational and activity of serum albumin. J. Nanosci. Nanotechnol. 18(11), 7818–7823 (2018)
Zhao, X.C., Liu, R.T., Chi, Z.X., Teng, Y., Qin, P.F.: new insights into the behavior of bovine serum albumin adsorbed onto carbon nanotubes: comprehensive spectroscopic studies. J. Phys. Chem. B 114(16), 5625–5631 (2010)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Sun, F., Yu, J. et al. Binding of esketamine to human serum albumin for clinical implications. J Incl Phenom Macrocycl Chem 101, 101–109 (2021). https://doi.org/10.1007/s10847-021-01090-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-021-01090-6