Abstract
The binding properties of cyclohexanocucurbit[6]uril (Cy6Q[6]) host toward three 1,w-bisbenzimidazolyl derivatives (guests 1–3, with alkyl chain of different lengths as linker) have been analyzed by 1H NMR spectroscopy and isothermal titration calorimetry (ITC) in aqueous solution and X-ray crystallography in solid state. The 1H NMR spectroscopy reveal that all guests can form 1:1 and 1:2 inclusion complexes with Cy6Q[6] macrocyles residing over benzoimidazole groups. The actual binding ratios or modes depend on the amounts of the host. Interestingly, the encapsulation and release of the guests can be controlled through the pH values of the solution. ITC data show that the binding process of host Cy6Q[6] with gusts 1–3 is driven by enthalpy, which benefits from hydrophobic effects and host–guest interactions. X-ray diffraction analysis provide unambiguous evidence that the benzoimidazole group of the guests 1 and 2 can be encapsulated into the Cy6Q[6] cavity, forming 1:1 host–guest inclusion complexes. The formation of these 1:1 binary inclusion complexes is attributed to the cooperativity of ion–dipole interaction, van der Waals interaction, C–H···π interaction, and hydrogen-bonding interaction.
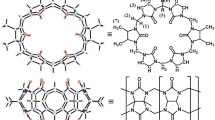
Graphic abstract
Binding interactions of 1,ω-bisbenzimidazolyl derivatives (guests) with cyclohexanocucurbit[6]uril (Cy6Q[6]) both in aqueous solution and solid state have been investigated by various tools. The results reveal that all guests can form 1:1 or 1:2 inclusion complexes with Cy6Q[6] residing over benzoimidazole groups of the guests.









Similar content being viewed by others
References
Atwood, J.L., Steed, J.W.: Encyclopaedia of supramolecular chemistry. Taylor & Francis, New York (2004)
Kolesnichenko, I.V., Anslyn, E.V.: Practical applications of supramolecular chemistry. Chem. Soc. Rev. 46, 2385–2390 (2017)
Liu, Z., Nalluri, S.K.M., Stoddart, J.F.: Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 46, 2459–2478 (2017)
Zhao, D., Moore, J.S.: Shape-persistent arylene ethynylene macrocycles: syntheses and supramolecular chemistry. Chem. Commun. 2003(7), 807–818 (2003)
Zhang, W., Moore, J.S.: Shape-persistent macrocycles: structures and synthetic approaches from arylene and ethynylene building blocks. Angew. Chem. Int. Ed. 45, 4416–4439 (2006)
Jin, Y., Zhang, A., Huang, Y., Zhang, W.: Recent advances in dynamic covalent chemistry. Chem. Commun. 46, 8258–8260 (2010)
Oshovsky, G.V., Reinhoudt, D.N., Verboom, W.: Supramolecular chemistry in water. Angew. Chem. Int. Ed. 46, 2366–2393 (2007)
Harada, A., Takashima, Y., Nakahata, M.: Supramolecular polymeric materials via cyclodextrin–guest interactions. Acc. Chem. Res. 47, 2128–2140 (2014)
Appel, E.A., Barrio, J., Loh, X.J., Scherman, O.A.: Supramolecular polymeric hydrogels. Chem. Soc. Rev. 41, 6195–6214 (2012)
Yu, G.C., Jie, K.C., Huang, F.H.: Supramolecular amphiphiles based on host–guest molecular recognition motifs. Chem. Rev. 115, 7240–7303 (2015)
Murray, J., Kim, K., Ogoshi, T., Yao, W., Gibb, B.C.: The aqueous supramolecular chemistry of cucurbit[n]urils, pillar[n]arenes and deep-cavity cavitands. Chem. Soc. Rev. 46, 2479–2496 (2017)
Lagona, J., Mukhopadhyay, P., Chakrabarti, S., Isaacs, L.: The cucurbit[n]uril family. Angew. Chem. Int. Ed. 44, 4844–4870 (2005)
Kaifer, A.E.: Toward reversible control of cucurbit[n]uril complexes. Acc. Chem. Res. 47, 2160–2167 (2014)
Masson, E., Ling, X., Joseph, R., Kyeremeh-Mensah, L., Lu, X.: Cucurbituril chemistry: a tale of supramolecular success. RSC Adv. 2, 1213–1247 (2012)
Assaf, K.I., Nau, W.M.: Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 44, 394–418 (2015)
Ni, X.L., Xiao, X., Cong, H., Liang, L.L., Chen, K., Chen, X.J., Ji, N.N., Zhu, Q.J., Xue, S.F., Tao, Z.: Cucurbit[n]uril-based coordination chemistry: from simple coordination complexes to novel poly-dimensional coordination polymers. Chem. Soc. Rev. 42, 9480–9508 (2013)
Barrow, S.J., Kasera, S., Rowland, M.J., Barrio, J., Scherman, O.A.: Cucurbituril-based molecular recognition. Chem. Rev. 115, 12320–12406 (2015)
Jon, S.Y., Selvapalam, N., Oh, D.H., Kang, J.K., Kim, S.Y., Jeon, Y.J., Lee, J.W., Kim, K.: Facile synthesis of cucurbit[n]uril derivatives via direct functionalization: expanding utilization of cucurbit[n]uril. J. Am. Chem. Soc. 125, 10186–10187 (2003)
Wu, F., Wu, L.H., Xiao, X., Zhang, Y.Q., Xue, S.F., Tao, Z., Day, A.I.: Locating the cyclopentano cousins of the cucurbit[n]uril family. J. Org. Chem. 77, 606–611 (2012)
Zhao, Y.J., Xue, S.F., Zhu, Q.J., Tao, Z., Zhang, J.X., Wei, Z.B., Long, L.S., Hu, M.L., Xiao, H.P., Day, A.I.: Synthesis of a symmetrical tetrasubstituted cucurbit[6]uril and its host-guest inclusion complex with 2,2′-bipyridine. Chin. Sci. Bull. 49, 1111–1116 (2004)
Zhao, J., Kim, H.J., Oh, J., Kim, S.Y., Lee, J.W., Sakamoto, S., Yamaguchi, K., Kim, K.: Cucurbit[n]uril derivatives soluble in water and organic solvents. Angew. Chem. Int. Ed. 40, 4233–4235 (2001)
Vinciguerra, B., Cao, L.P., Cannon, J.R., Zavalij, P.Y., Fenselau, C., Isaacs, L.: Synthesis and self-assembly processes of monofunctionalized cucurbit[7]uril. J. Am. Chem. Soc. 134, 13133–13140 (2012)
Singla, P., Luxami, V., Paul, K.: Benzimidazole-biologically attractive scaffold for protein kinase inhibitors. RSC Adv. 4, 12422–12440 (2014)
Maiti, B., Chanda, K.: Diversity oriented synthesis of benzimidazole-based biheterocyclic molecules by combinatorial approach: a critical review. RSC Adv. 6, 50384–50413 (2016)
Kim, M.O., Blachly, P.G., Kaus, J.W., McCammon, J.A.: Protocols utilizing constant pH molecular dynamics to compute pH-dependent binding free energies. J. Phys. Chem. B 119, 861–872 (2015)
Barooah, N., Mohanty, J., Bhasikuttan, A.C.: pH-Mediated stoichiometric switching of cucurbit[8]uril-hoechst-33258 complexes. J. Phys. Chem. B 117, 13595–13603 (2013)
Barooah, N., Sundararajan, M., Mohanty, J., Bhasikuttan, A.C.: Synergistic effect of intramolecular charge transfer toward supramolecular pKa shift in cucurbit[7]uril encapsulated coumarin dyes. J. Phys. Chem. B 118, 7136–7146 (2014)
Pischel, U., Uzunova, V.D., Remon, P., Nau, W.M.: Supramolecular logic with macrocyclic input and competitive reset. Chem. Commun. 46, 2635–2637 (2010)
Barooah, N., Mohanty, J., Pal, H., Bhasikuttan, A.C.: Supramolecular assembly of hoechst-33258 with cucurbit[7]uril macrocycle. Phys. Chem. Chem. Phys. 13, 13117–13126 (2011)
Ge, J.Y., Xue, S.F., Zhu, Q.J., Tao, Z., Zhang, J.X.: Interaction of cucurbit[n = 6 ~ 8]urils and benzimidazole derivatives. J. Incl. Phenom. Macro. Chem. 58, 63–69 (2007)
Mukhopadhyay, C., Ghosh, S., Schmiedekamp, A.M.: Unraveling the molecular recognition of “three methylene spacer” bis(benzimidazolium) moiety by dibenzo-24-crown-8: pseudorotaxanes under study. Org. Biomol. Chem. 10, 1434–1439 (2012)
Zhu, K., Vukotic, V., Noujeim, N., Loeb, S.J.: Bis(benzimidazolium) axles and crown ether wheels: a versatile templating pair for the formation of [2]rotaxane molecular shuttles. Chem. Sci. 3, 3265–3271 (2012)
Li, L., Clarkson, G.J.: New bis(benzimidazole) cations for threading through dibenzo-24-crown-8. Org. Lett. 9, 497–500 (2007)
Ghosh, S., Schmiedekamp, A.M., Mukhopadhyay, C.: Bis(benzimidazolium)methane salts: a potential guest for dibenzo-24-crown-8 towards [2]pseudorotaxanes. Tetrahedron 68, 9826–9835 (2012)
Ni, X.L., Yi, J.M., Song, S., Zhang, Y.Q., Xue, S.F., Zhu, Q.J., Tao, Z.: Supramolecular interactions of bisbenzimidazolyl derivatives with cucurbit[7]uril, potential axle molecules bearing a novel fluorescent signal response. Tetrahedron 69, 6219–6222 (2013)
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009)
Palatinus, L., Chapuis, G.: SUPERFLIP-a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 40, 786–790 (2007)
Palatinus, L., Prathapa, S.J., Smaalen, S.: EDMA: a computer program for topological analysis of discrete electron densities. J. Appl. Crystallogr. 45, 575–580 (2012)
Sheldrick, G.M.: A short history of SHELX. Acta Crystallogr. Sect. A 64, 112–122 (2008)
Sheldrick, G.M.: Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 71, 3–8 (2015)
Spek, A.L.: Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 65, 148–155 (2009)
Biedermann, F., Uzunova, V.D., Scherman, O.A., Nau, W.M., Simone, A.D.: Release of high-energy water as an essential driving force for the high-affinity binding of cucurbit[n]urils. J. Am. Chem. Soc. 134, 15318–15323 (2012)
Biedermann, F., Schneider, H.J.: Experimental binding energies in supramolecular complexes. Chem. Rev. 116, 5216–5300 (2016)
Rebek Jr., J.: Molecular behavior in small spaces. Acc. Chem. Res. 42, 1660–1668 (2009)
Ajami, D., Rebek Jr., J.: More chemistry in small spaces. Acc. Chem. Res. 46, 990–999 (2013)
Lin, R.L., Li, J.Q., Liu, J.X., Kaifer, A.E.: The binding interactions between cyclohexanocucurbit[6]uril and alkyl viologens give rise to a range of diverse structures in the solid and the solution phases. J. Org. Chem. 80, 10505–10511 (2015)
Fang, G.S., Sun, W.Q., Zhao, W.X., Lin, R.L., Tao, Z., Liu, J.X.: Host–guest complexation of di-cyclohexanocucurbit[6]uril and hexa-cyclohexano -cucurbit[6]uril with alkyldiammonium ions: a comparative study. Org. Biomol. Chem. 14, 674–679 (2016)
Li, Q., Qiu, S.C., Chen, K., Zhang, Y., Wang, R., Huang, Y., Tao, Z., Zhu, Q.-J., Liu, J.X.: Encapsulation of alkyldiammonium ions within two different cavities of twisted cucurbit[14]uril. Chem. Commun. 52, 2589–2592 (2016)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21371004), Natural Science Foundation of Anhui Province of China (1808085MB43) and the Key scientific research projects in Colleges and Universities of Henan Province (Grant No. 16A180026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, LM., Zhang, K., Lin, RL. et al. Binding interactions of bisbenzimidazolyl derivatives with cyclohexanocucurbit[6]uril. J Incl Phenom Macrocycl Chem 96, 125–135 (2020). https://doi.org/10.1007/s10847-019-00957-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-019-00957-z