Abstract
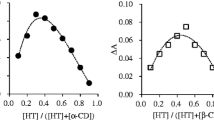
The formation of inclusion complexes between betahistine (BTH) and the β-CD derivatives were investigated. The binding constant (K c ) of the BTH/β-cyclodextrin (β-CD) inclusion complex was determined to be 1400 L/mol based on UV data. The structure of the BTH/β-CD complex in aqueous solution was examined by 1H–1H rotating frame nuclear Overhauser effect spectroscopy (ROESY) NMR, and the pyridine ring and side chain moiety of the BTH molecule were found to be inserted from the secondary hydroxyl face of the β-CD. The thermal properties of the solid BTH/β-CD inclusion complexes prepared by kneading and freeze-drying methods were studied by differential scanning calorimetry, and for comparison, solid betahistine mesilate (BTHm)/β-CD inclusion complexes were similarly prepared. Humidity tests showed that the solid BTH/β-CD complexes exhibited less moisture absorption compared to the BTHm alone and BTHm/β-CD complexes.








Similar content being viewed by others
References
Elia, J.C.: Double-blind evaluation of a new treatment for Ménière’s syndrome. JAMA 196, 187–189 (1966)
Horton, B.T., Von Leden, H.: Clinical use of beta-2-pyridylalkylamines part 1. Proceedings of the staff meetings. Mayo Clin. 37, 692–702 (1962)
Saenger, W.: Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Intl. Ed. Engl. 19, 344–362 (1980)
Wenz, G.: Cyclodextins as building blocks for supramolecular structures and functional units. Angew. Chem. Intl. Ed. Engl. 33, 803–822 (1994)
Uekama, K.: Novel approach of cyclodextrin-based pharmaceutical formulation. Yakugaku Zasshi: J.Pharm. Soc. Jpn. 132(1), 85–105 (2012)
Loukas, Y.L., Vraka, V., Gregoriadis, G.: Drugs, in cyclodextrins, in liposomes: a novel approach to the chemical stability of drugs sensitive to hydrolysis. Intl. J. Pharm. 162(1–2), 137–142 (1998)
Ong, J.K., Sunderland, V.B., Mcdonald, C.: Influence of hydroxypropyl β-cyclodextrin on the stability of benzylpenicillin in chloroacetate buffer. J. Pharm. Pharmacol. 49(6), 617–621 (1997)
Maeda, H., Onodera, T., Nakayama, H.: Inclusion complex of α-lipoic acid and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 68, 201–206 (2010)
Maeda, H., Ogawa, Y., Nakayama, H.: Inclusion complexes of melatonin and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 78, 217–224 (2013)
Maeda, H., Tanaka, R., Nakayama, H.: Inclusion complexes of trihexyphenidyl with natural and modified cyclodextrins. 4, p. 218. SpringerPlus, London (2015)
Frijlink, H.W., Eissens, A.C., Schoonen, A.-J.M., Lerk, C.F.: The effects of cyclodextrins on drug release from fatty suppository bases. part 1. In vitro observations. Eur. J. Pharm. Biopharm. 37(3), 178–182 (1991)
Chauhan, R., Madan, J., Kaushik, D., Sardana, S., Pandey, R.S., Sharma, R.: Inclusion complex of colchicine in hydroxypropyl-β-cyclodextrin tenders better solubility and improved pharmacokinetics. Pharma. Devel. Technol. 18(2), 313–322 (2013)
Liu, M., Cao, W., Sun, Y., He, Z.: Preparation, characterization and in vivo evaluation of formulation of repaglinide with hydroxylpropyl-β-cyclodextrin. Intl. J. Pharm. 477(1–2), 159–166 (2014)
Lindner, K., Szente, L., Szejtli, J.: Food flavouring with β-cyclodextrin-complexed flavour substances. Acta Aliment. 10(3), 175–186 (1981)
Szente, L., Szejtli, J.: Molecular encapsulation of natural and synthetic coffee flavor with β-cyclodextrin. J. Food Sci. 51(4), 1024–1027 (1986)
Furuta, T., Yoshii, H., Kobayashi, T., Nishitarumi, T., Yasunishi, A.: Powdery encapsulation of d-limonene by kneading with mixed powders of β-cyclodextrin and maltodextrin at low water content. Biosci. Biotech. Biochem. 58(5), 847–850 (1994)
Benesi, H.A., Hildebrand, J.H.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71(8), 2703–2707 (1949)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. App. 9, 113–203 (1928)
Rawat, S., Jain, S.K.: Stability enhancement of celecoxib using β-cyclodextrin inclusion complexes. Eur. J. Pharm. Biopharm. 57, 263–267 (2004)
Acknowledgments
The authors thank Assistant Professor C. Tode of Kobe Pharmaceutical University for the measurements of 1H–1H COSY and 1H–1H ROESY NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, H., Iga, Y. & Nakayama, H. Characterization of inclusion complexes of betahistine with β-cyclodextrin and evaluation of their anti-humidity properties. J Incl Phenom Macrocycl Chem 86, 337–342 (2016). https://doi.org/10.1007/s10847-016-0658-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-016-0658-4