Abstract
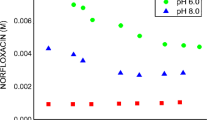
Inclusion complexes using α-, β-, γ-, and hydroxypropyl-β-CD (HP-β-CD) were produced with the antibiotic enrofloxacin, with the aim of increasing its solubility by complexation. Phase solubility diagrams were obtained, to confirm the formation of inclusion complexes, and to determine the solubility enhancement and stability constant of each complex. Enrofloxacin inclusion in β-CD showed the highest value of the complex stability constant (35.56 mmol L−1), but the greatest increase in solubility was obtained using HP-β-CD reaching a 1258% increase over enrofloxacin solubility in the absence of CD. The order of highest enrofloxacin solubility achieved was: HP-β-CD > α-CD > γ-CD > β-CD. In addition, formation of complexes was confirmed by differential scanning calorimetry and thermogravimetry, applied to the complexes obtained by the kneading technique. The influence of citric acid, alone or as an adjunct of β-CD, on the solubility of enrofloxacin was also determined. A solution of 15 mmol L−1 citric acid dissolved 10 g L−1 of enrofloxacin, but a gradual increase in β-CD concentration in the presence of citric acid did not increase the degree of solubilization of enrofloxacin.






Similar content being viewed by others
References
Baluja, S., Bhalodia, R., Bhatt, M., Vekariya, N., Gajera, R.: Solubility of enrofloxacin sodium in various solvents at various temperatures. J. Chem. Eng. Data 53, 2897–2899 (2008)
Seedher, N., Agarwal, P.: Various solvent systems for solubility enhancement of enrofloxacin. Indian J. Pharm. Sci. 71, 82–87 (2009)
Tiwari, G., Tiwari, R., Rai, A.K.: Cyclodextrins in delivery systems: applications. J. Pharm. Bioall. Sci. 2, 72–78 (2010)
Grunenberg, A., Bothe, C., Keil, B.: Patent US 2011/0003829A1 (2011)
Matioli, G., Zanin, G.M., de Moraes, F.F.: Influence of substrate and product concentrations on the production of cyclodextrins by CGTase of Bacillus firmus, strain no. 37. Appl. Biochem. Biotechnol. 98–100, 947–961 (2002)
Del Vale, E.M.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Moriwaki, C., Costa, G.L., Ferracini, C.N., de Moraes, F.F., Zanin, G.M., Pineda, E.A.G., Matioli, G.: Enhancement of solubility of albendazole by complexation with β-cyclodextrin. Braz. J. Chem. Eng. 25, 255–267 (2008)
Challa, R., Ahuja, A., Ali, J., Khar, R.K.: Cyclodextrins in drug delivery: an updated review. A.A.P.S. Pharm. Sci. Tech. 6, E329–E357 (2005)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007)
Zhang, L., Wang, M., Shen, Y., Ma, Y., Luo, J.: Improvement of steroid biotransformation with hydroxypropyl-β-cyclodextrin induced complexation. Appl. Biochem. Biotechnol. 159, 642–654 (2009)
Higuchi, T., Connors, K.A.: Phase-solubility techniques. In: Reilley, C.N. (ed.) Advances in Analytical Chemistry and Instrumentation, vol. 4. Wiley Interscience, New York (1965)
Hirose, K.: A practical guide for the determination of binding constants. J. Incl. Phenom. Macrocycl. Chem. 39, 193–209 (2001)
Loftsson, T., Magnúsdóttir, A., Másson, M., Sigurjónsdóttir, J.F.: Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 91, 2307–2316 (2002)
Loftsson, T., Hreinsdóttir, D., Másson, M.: Evaluation of cyclodextrin solubilization of drugs. I. J. Pharm. 302, 18–28 (2005)
Melo, N.F.S., Grillo, R., Moraes, C.M., Brito, C.L., Trossini, G.H.G., Menezes, C.M.S., Ferreira, E.I., Rosa, A.H., Fraceto, L.F.: Preparação e caracterização inicial de complexo de inclusão entre nitrofurazona e 2-hidroxipropil-β-ciclodextrina. Rev. Ciênc. Farm. Básica Apl. 28, 35–44 (2007)
Szejtli, J.: Cyclodextrin Technology. Kluwer, Academic Publishers, Dordrecht (1988)
Lizondo, M., Pons, M., Gallardo, M., Estelrich, J.: Physicochemical properties of enrofloxacin. J. Pharm. Biomed. Anal. 15, 1845–1849 (1997)
Acknowledgments
The authors thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and Fundação Araucária for financial support, and Formil Química Ltda for the gift of enrofloxacin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calsavara, L.P.V., Zanin, G.M. & de Moraes, F.F. Enrofloxacin inclusion complexes with cyclodextrins. J Incl Phenom Macrocycl Chem 73, 219–224 (2012). https://doi.org/10.1007/s10847-011-0045-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-0045-0