Abstract
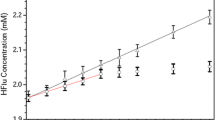
A detailed NMR (1H, COSY, and ROESY) spectroscopic study of complexation of Flunarazine (FL) with α- and β-CD was carried out. 1H NMR titration studies confirmed the formation of FL/α-CD and FL/β-CD complexes as evidenced by chemical shift variations of the proton resonances of both the CDs and FL. The stoichiometry of the complexes was determined to be 1:2 (FL/α-CD) and 1:1 (FL/β-CD) and overall binding constants were also calculated. It was confirmed with the help of ROESY spectral data that only one of the F-substituted aromatic ring and phenyl ring penetrate the α-CD cavity while both F-substituted aromatic rings as well as phenyl ring penetrates the β-CD cavity during complexation. The binding modes of FL/CD cavity interactions derived from ROESY experimental data show that the resulting complex of FL with β-CD possesses better induced fit interaction as compared to α-CD, which is responsible for the enhanced molecular stability with β-CD in comparison to α-CD. The mode of penetration of guest into the CD cavity and structures of the complexes has been established.











Similar content being viewed by others
References
Berk, M., Kirchmann, N.H.: Enhanced blockade of 45Ca2+ uptake into platelets in manic patients with bipolar affective disorder with flunarazine and verapamil. Human Psychopharmacol. Clin. Exp. 10, 299–303 (1995)
Albani, F., Baldrati, A., Cortelli, P., Riva, R., Baruzzi, A.: Flunarizine plasma concentrations and side effects in migraine patients. Headache J. Head Face Pain 30, 369–370 (2005)
Bender, M.L., Komiyama, M.: Cyclodextrin Chemistry. Springer-Verlag, NewYork (1978)
Steed, J.W., Atwood, J.L.: Supramolecular Chemistry. Wiley-VCIL, Weinchim (2000)
Szejtli, J.: Cyclodextrin Technology. Kluwer Academic, Dordrecht (1998)
Esclusa-Diaz, M.T., Gayo-Otero, M., Perez-Marcos, M.B., Villa-Jato, J.L.: Preparation and evolution of ketoconazole- β-cyclodextrin multicomponent complexes. Int. J. Pharm. 142, 183–187 (1996)
Obaidat, A.A., Matalgah, S.M., Najib, N.M.: Improvement and characterization of the in vitro dissolution behavior of sulindac by complexation with β-cyclodextrin. Acta Pharm. 52, 9–18 (2002)
Fenyvesi, E., Szente, L., Russell, N.R., McNamara, M.: Specific Guest Types. In Comprehensive Supramolecular Chemistry. Elsevier Science Ltd, New York (1996)
Monti, S., Sortino, S., De Guidi, G., Marconi, G.: Supramolecular photochemistry of 2-(3-benzoylphenyl)propionic acid (Ketoprofen). A study in the -cyclodextrin cavity. New J. Chem. 22, 599–604 (1998)
Jimenez, M.C., Miranda, M.A., Tormos, R.: Photodecarbylation of 2-phenylpropionic acid in solution and included within β-cyclodextrin. Tetrahedron 51, 2953–2958 (1995)
Schneider, H.J., Hacket, F., Rudiger, V., Ikeda, H.: NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 98, 1755–1785 (1998), and references cited therein
Laverde Jr, A., Conceicao, G., Queiroz, S., Fujiwara, F., Marsaioli, A.: An NMR tool for cyclodextrin selection in enantiomeric resolution by high-performance liquid chromatography. Magn. Res. Chem. 40, 433–442 (2002)
Neuhaus, D., Williamson, M.: The Nuclear Overhauser Effect in Structural and Conformational Analysis. VCH Publishers, New York (1989)
Komiyama, M., Hirai, H.: Time averaged conformation of the inclusion complexes of β-cyclodextrin with tert-butylphenols. Chem. Lett. 12, 1467–1470 (1980)
Rekharsky, M.V., Goldberg, R.N., Schwarz, F.P., Tiwari, Y.B., Ross, P.D., Yamashoji, Y., Inoue, Y.: Thermodynamic and nuclear magnetic resonance study of the interactions of alpha- and beta-cyclodextrin with model substances: phenethylamine, ephedrines, and related substances. J. Am. Chem. Soc. 117, 8830–8840 (1995)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113–203 (1928)
Scott, R.L.: Some comments on the Benesi-Hildebrand equation. Rec. Trav. Chim. 75, 787–789 (1956)
Benesi, H.A., Hildebrand, J.H.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71, 270–271 (1949)
Bother-By, A.A., Stephens, R.L., Lee, J.J.: Structure determination of a tetrasaccharide: transient nuclear Overhauser effects in the rotating frame. J. Am. Chem. Soc. 106, 811–813 (1984)
Acknowledgements
Flunarizine and β-cyclodextrin were very kindly provided by Dr. Reddy’s laboratory India, and Geertrui Haest, Cerestar Cargill, Belgium, respectively. We are highly grateful to Prof. S. W. Homans, Department of Biochemistry and Molecular Biology, University of Leeds, UK and Dr. P. De Waard, Department of Biophysics, Dreijenlaan, The Netherlands, for their help in obtaining some of the NMR data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maheshwari, A., Sharma, D. A comparative study of inclusion complexes of flunarizine with alpha (α-CD) and beta-cyclodextrin (β-CD). J Incl Phenom Macrocycl Chem 68, 453–459 (2010). https://doi.org/10.1007/s10847-010-9809-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9809-1